A kind of pyrimidine derivative and its preparation method and application
A pyrimidine and drug technology, applied in the field of pyrimidine derivatives and their preparation, can solve the problems of no compound, large toxic and side effects, short half-life and the like, and achieve the effects of reducing drug dosage, good sustained release effect and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1, 1-(β-L-ribofuranose)-5-amino-pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (compound 1)
[0042]
[0043] Among them, Ac represents an acetyl group.
[0044] 1-(2',3',5'-O-acetyl-β-L-ribofuranose)-5-amino-pyrimido[4,5-d]pyrimidine-2,4(1H,3H)- Diketone 120mg was dissolved in 10mL 0.1M NaOMe / MeOH, and reacted at 75°C for 20 minutes. After cooling to room temperature (25°C), neutralized with diluted glacial acetic acid, a precipitate was formed, filtered, and the precipitate was used Washed three times with methanol, washed once with water, and dried under vacuum to obtain white powder 1-(β-L-ribofuranose)-5-amino-pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione (70 mg, 82% yield, 99% purity).
[0045] 1-(β-L-ribofuranose)-5-amino-pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione:
[0046] 1 H NMR (DMSO-d 6 ,600MHz): δ3.55-3.63(1H,m,5'-H),3.76-3.79(1H,m,5'-H β ),3.92-3.96(1H,m,4′-H),4.07-4.09(1H,3′-H),4.13-4.15(1H,2′-H),5.09-5.11(1H,5′-OH ),5.26-5.30(t,1H,3′-OH),5.55-...
Embodiment 2
[0050] Embodiment 2, compound 1 forms nanoparticle structure by self-assembly
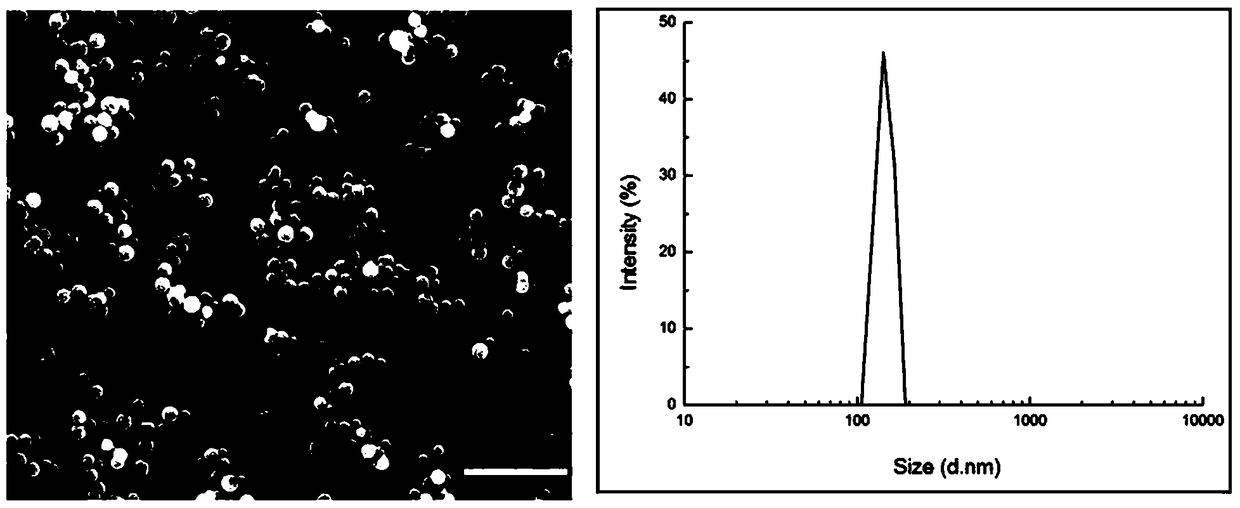
[0051] A certain amount of compound 1 was dissolved in water, heated to boiling, cooled, and left to stand at room temperature for 24 hours; the results of scanning electron microscopy (SEM) and dynamic laser light scattering (DLS) showed that compound 1 could form particles by self-assembly in aqueous solution. Granular nanostructures with uniform diameter (~150nm), such as figure 1 shown.
Embodiment 3
[0052] Example 3. Nanoparticles formed by self-assembly of compound 1 are used to encapsulate drug 5-fluorouracil
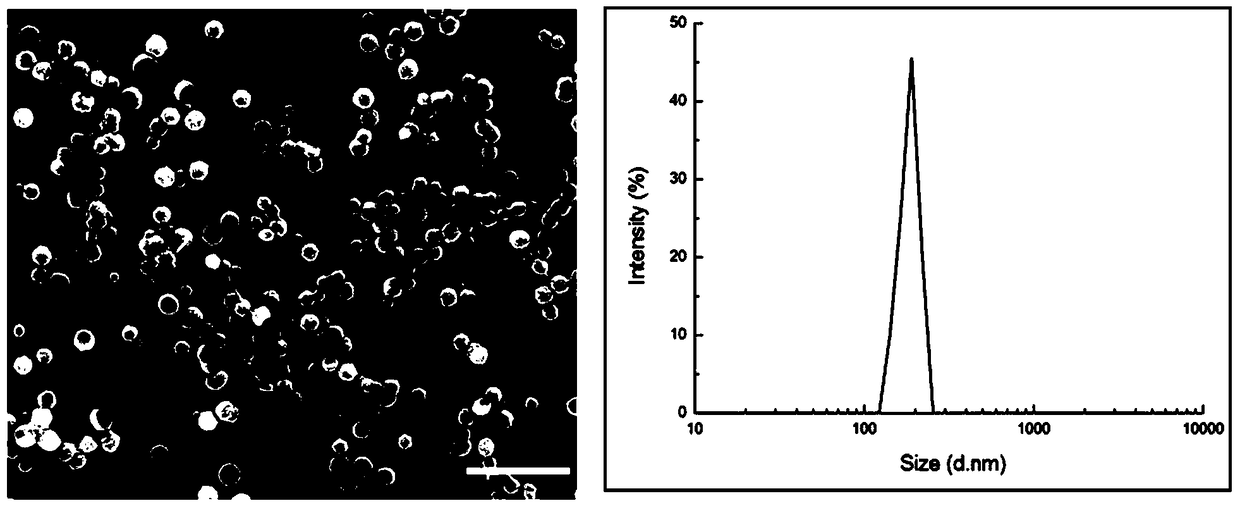
[0053] A certain amount of compound 1 was dissolved in PBS, heated to about 100°C, cooled to room temperature, and left to stand for 24 hours, then added with a certain amount of 5-fluorouracil, and then left to stand for another 24 hours; the results of scanning electron microscopy and dynamic laser light scattering after the drug was wrapped It shows that compound 1 of the present invention can effectively wrap 5-fluorouracil to form a granular structure with uniform particle size, such as figure 2 shown.
[0054] In order to illustrate the beneficial effects of the present invention, the present invention provides the following test examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com