Application of Cephaloziellins H in preparation of prostate cancer treating medicine
A technology of cephaloziellinsh and 1.cephaloziellinsh, which can be applied in the directions of drug combination, antitumor drug, medical formula, etc., can solve the problem that there is no report on the activity of prostate cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
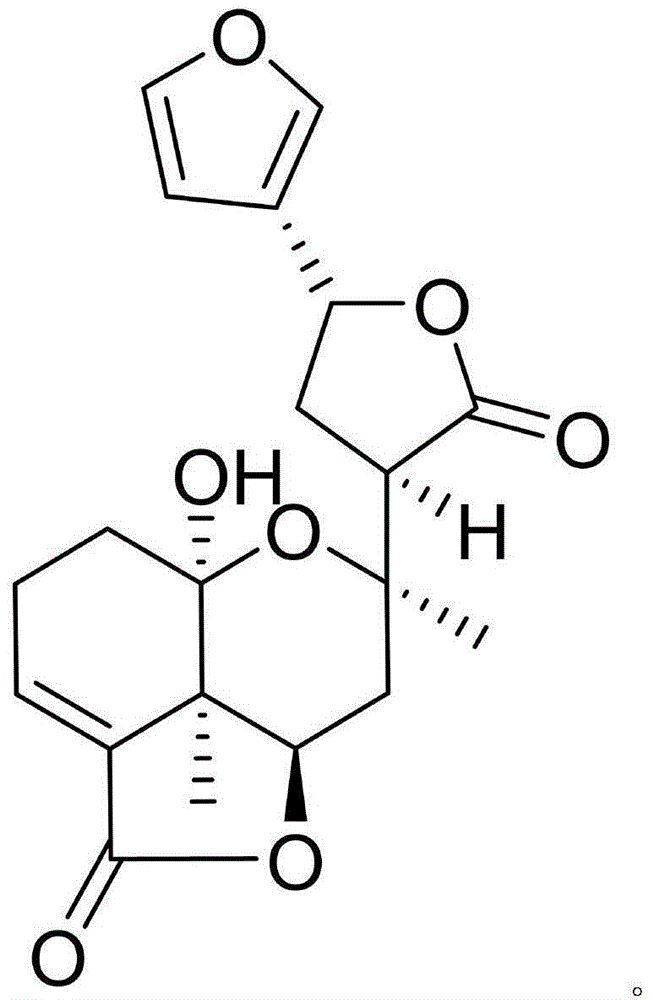
[0012] Example 1: Isolation, Preparation and Structure Confirmation of CephaloziellinsH
[0013] The preparation method of Cephaloziellins H is the same as that reported in the literature (Secondary Metabolites from the Chinese Liverwort Cephaloziellakiaeri, J. Nat. Prod., 2013, 76, 1700-1708).
[0014] Confirmation of structure: colorless crystal (methanol), melting point 150-153°C. According to HR-ESI-MS, the molecular formula is C 20 h 22 o 7 , with an unsaturation of 10. H NMR spectrum data δ H (ppm, DMSO-d 6 , 600MHz): H-1 (1.81, m), H-1 (1.65, m), H-2 (2.23, 2H, m), H-3 (6.74, dd, J=3.6, 3.0), H- 6 (4.62, dd, J=10.2, 6.6), H-7 (1.95, dd, J=13.8, 6.6), H-7 (2.08, dd, J=13.8, 10.2), H-9 (2.48, dd , J=9.0, 7.2), H-11 (2.53, dt, J=13.0, 7.2), H-11 (2.19, ddd, J=13.0, 9.0, 6.0), H-12 (5.36, dd, J= 7.2, 6.0), H-14 (6.27, br, s), H-15 (7.33, br, s), H-16 (7.35, br, s), H-17 (1.42, s), H-19 (1.26, s); C NMR spectrum data δ C (ppm, DMSO-d 6 , 150Hz): 33.3 (CH 2 , 1-C)...
Embodiment 2
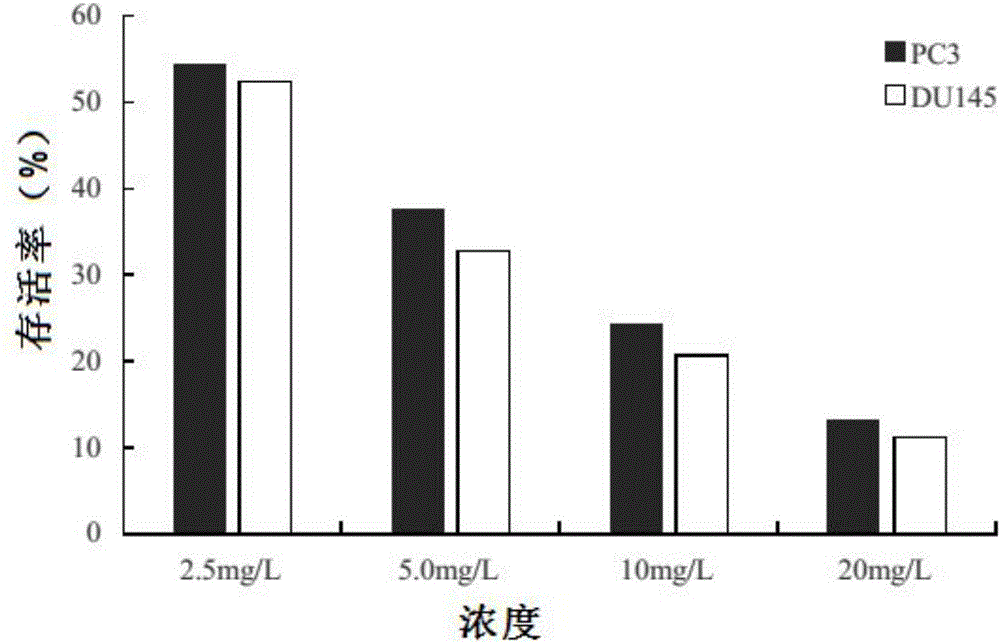
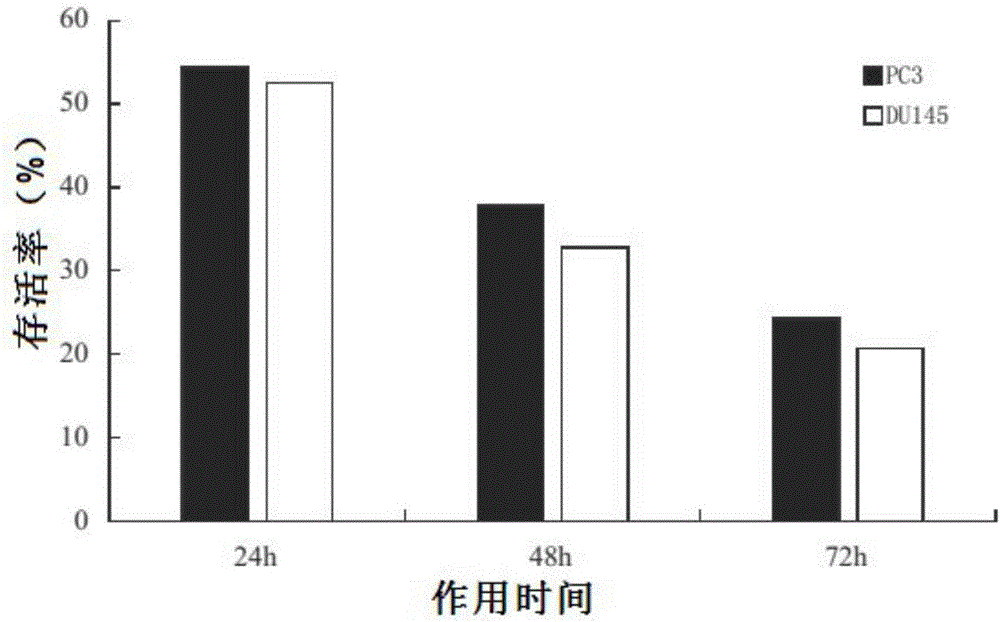
[0015] Embodiment 2: Pharmacological action test of Cephaloziellins H
[0016] 1. Materials and Instruments
[0017] Prostate cancer cell line PC3 (ArCC-CRL-1435), prostate cancer cell line DU145 (ATCC-HTB-81). CephaloziellinsH is self-made, and the HPLC normalized purity is greater than 98%. It is prepared into a storage solution with a concentration of 1.0 g / L with dimethylsulfoxide (DMSO) for future use. The CCK-8 kit is a product of Japan Tongren Chemical Research Institute. RPMI-1640 medium was purchased from Gibco. Fetal bovine serum (FBS) was purchased from Hyelone Company. Penicillin / streptomycin is a product of Shanghai Pioneer Pharmaceuticals. Trypsin was purchased from Huamei Bioengineering Company. Dimethyl sulfoxide (DMSO) was purchased from Shanghai Huashun Bioengineering Company. Agarose (Agarose), dithiothreitol (DTT), phenylmethylsulfonphthalein fluoride (PMSF), and tetramethylethylenediamine (TEMED) are products of Sigma. Sodium dodecylsulfonate (SDs),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com