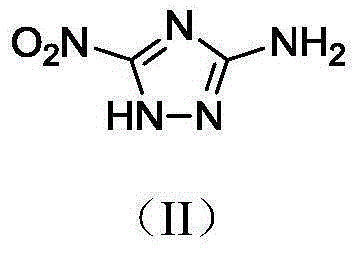
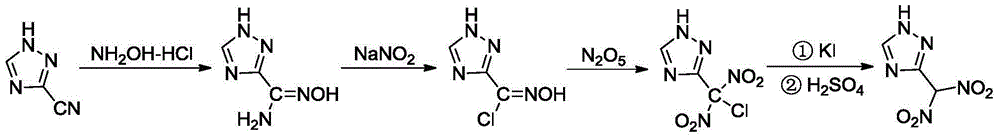
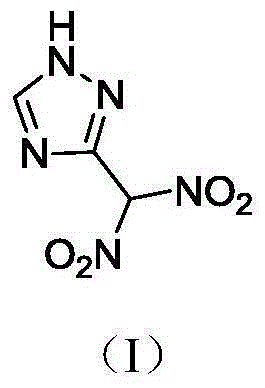
3-gem methyl dinitro-1,2,4-triazole compound
A technology of gem-dinitromethyl and triazole compounds, applied in the field of energetic materials, can solve the problems of low energy and low compound density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of 3-amidoxime-1,2,4-triazole
[0026] Under stirring, 25.0g (0.27mol) of 3-cyano-1,2,4-triazole, 230.0mL of distilled water, 24.0g (0.35mol) of hydroxylamine hydrochloride were added to the flask in turn, and 33.9g of 41.0% ( mass) of sodium hydroxide aqueous solution, after the dropwise addition, the temperature was raised to 60°C for 2.5 hours, the reaction liquid was cooled to room temperature, filtered, washed and dried to obtain 28.3g of 3-amidoxime-1,2,4-triazole , and the yield was 83.8%.
[0027] Structure Identification:
[0028] Infrared spectrum: IR (KBr, cm-1), υ: 3472, 3453, 3360, 3336 (-NH 2 ), 3143 (N-OH), 1655 (C=N), 932 (N-O), 1472, 1390, 1292, 1151 (triazole ring)
[0029] NMR spectrum: 1 HNMR (DMSO-d 6 ,500MHz), δ: 5.831(s,2H,NH 2 ),8.323(s,1H,NH),9.956(s,1H,CH),14.281(s,1H,OH); 13 CNMR (DMSO-d 6 ,125MHz), δ: 143.79, 147.76, 151.84
[0030] Elemental analysis: Molecular formula C 3 h 5 N 5 o
[0031] Theoretical value: C28....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com