Application of miRNA to diagnosis and treatment of acute myelogenous leukemia
An acute myeloid and leukemia technology, applied in the application field of miRNA-1277 in the diagnosis and treatment of acute myeloid leukemia, can solve the problems of inducing cancer and abnormal gene expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
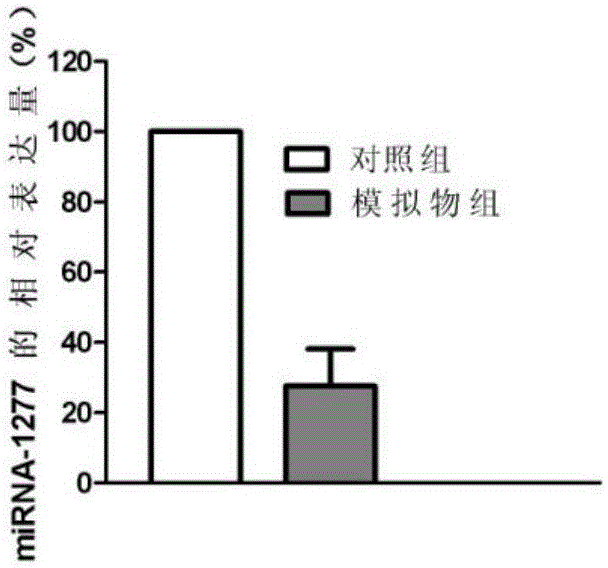
[0046] Example 1 Screening of miRNAs associated with acute myeloid leukemia
[0047] 1. Sample acquisition: Collect 10 blood samples from healthy people and blood samples from patients with acute myeloid leukemia. The acquisition of all the above samples was approved by the ethics committee of the organization.
[0048] 2. Extraction of total RNA from samples
[0049] Total RNA was extracted using the BloodRNA extraction kit from U-gene. Specific steps are as follows:
[0050] 1) Add 5 times the volume of 1×XR-I buffer per volume of fresh blood (maximum 1ml), for example: add 5ml of XR-I buffer per 1ml of blood, and vortex to mix;
[0051] 2) Ice-bathed for 15 minutes, quickly mixed twice on a vortex shaker, the solution became clear, indicating that the red blood cells had been lysed. If the hematocrit or ECR of individual samples increases, extend the ice bath time to 20 minutes;
[0052] 3) Centrifuge at 450g for 10min at 4°C to precipitate white blood cells, and compl...
Embodiment 2
[0078] Example 2 QPCR verification of differentially expressed miRNA-1277
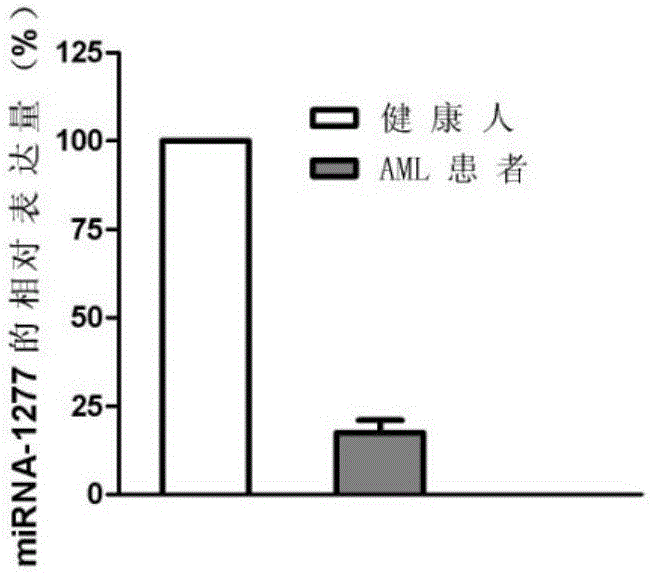
[0079] 1. According to the detection results of the miRNA chip, select miRNA-1277 for large sample QPCR verification. According to the sample collection method in Example 1, 80 blood samples from patients with acute myeloid leukemia and 80 blood samples from healthy people were selected.
[0080] 2. The RNA extraction process is the same as in Example 1.
[0081] 3. Reverse transcription:
[0082]1) Prepare the reaction system according to Table 1
[0083] Table 1 reaction system
[0084] RNA template
1μg
10× buffer
2μl
dATP (10mM)
2μl
polyA polymerase
0.5μl
RNase inhibitor
0.5μl
wxya 2 o
Make up to 20μl
[0085] Incubate at 37°C for 1h.
[0086] 2) Add 1 μl of 0.5 μg / μl Oligo(dT) specific RT primer to the reaction tube, and incubate at 70° C. for 5 minutes.
[0087] 3) Immediately incubate on ice for 2 minutes to break ...
Embodiment 3
[0104] Example 3 Expression of miRNA-1277 in acute myeloid leukemia cell lines
[0105] 1. Cell culture
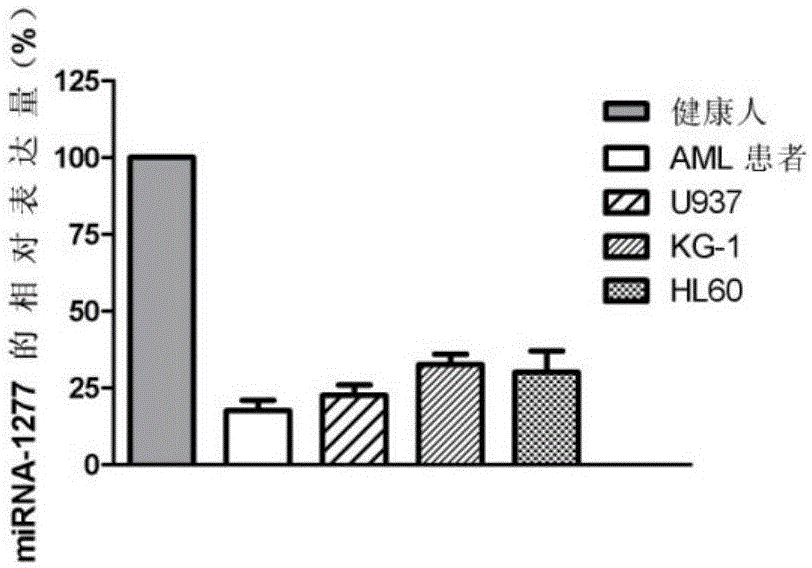
[0106] Acute myeloid leukemia cell lines U937, KG-1, HL-60 and bone marrow mononuclear cells isolated from AML patients and healthy people were routinely cultured in RPMI1640 medium containing 10% fetal bovine serum, placed at 37°C, 5%CO 2 in the incubator.
[0107] 2. QPCR
[0108] 2.1 Extraction of total cellular RNA: Extraction of total cellular RNA was performed using the RNA extraction kit from QIAGEN, following the instructions in the instructions.
[0109] 2.2 QPCR: the steps are the same as in Example 2.
[0110] 3. Results
[0111] Such as figure 2 As shown, compared with the bone marrow mononuclear cells of healthy people, the expression of miRNA-1277 in the bone marrow mononuclear cells of acute myeloid leukemia cell lines U937, KG-1, HL-60 and AML patients was significantly decreased (P<0.05).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com