Oral medication path of somatostatin analogue polypeptide drug
A technology of somatostatin and drug delivery route, which is applied in the field of medicine, can solve the problems of short half-life and instability of somatostatin, and achieve the effect of increasing the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
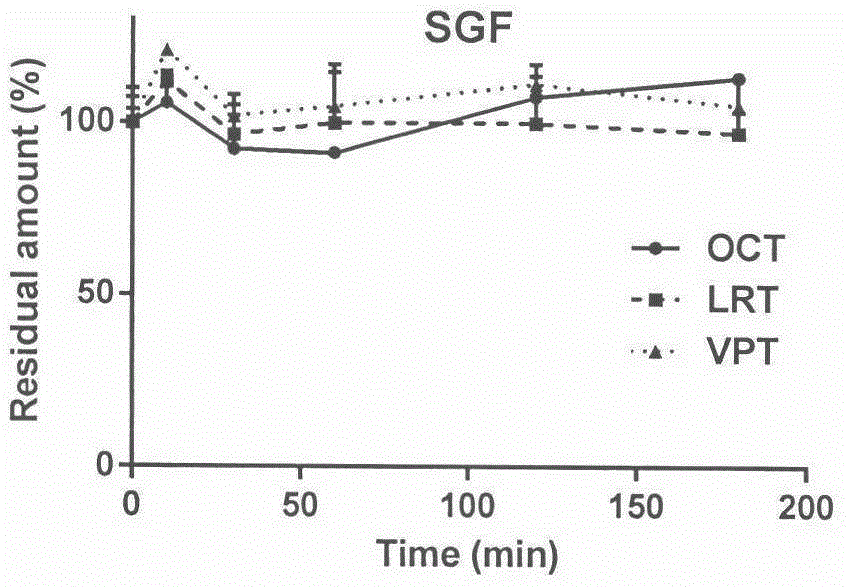
[0015] Embodiment 1 Stability of three kinds of somatostatin analogue polypeptides octreotide, vapreotide, and lanreotide in artificial gastric juice:
[0016] Step 1: Prepare artificial gastric juice according to the formula of the United States Pharmacopoeia. The specific formula is as follows: In 1000 mL of artificial gastric juice, 2.0 g of NaCl, 3.2 g of pepsin, and HCl were added to adjust the pH value to 1.2, and incubated at 37° C. for 30 minutes before use.
[0017] The second step: use the artificial gastric juice in the first step to prepare 1 mL of octreotide, vapreotide, and lanreotide solutions with a concentration of 100 μg / mL, and make 3 copies in parallel, and incubate at 37°C for a certain period of time (0, 10, 30, After 60, 120, and 180 minutes), the reaction was terminated with 3 mL of glacial acetonitrile, centrifuged at 30,000 g for 10 minutes, and the supernatant was taken for detection by LC-MS / MS.
[0018] Step 3: Calculate the residual amount. Taki...
Embodiment 2
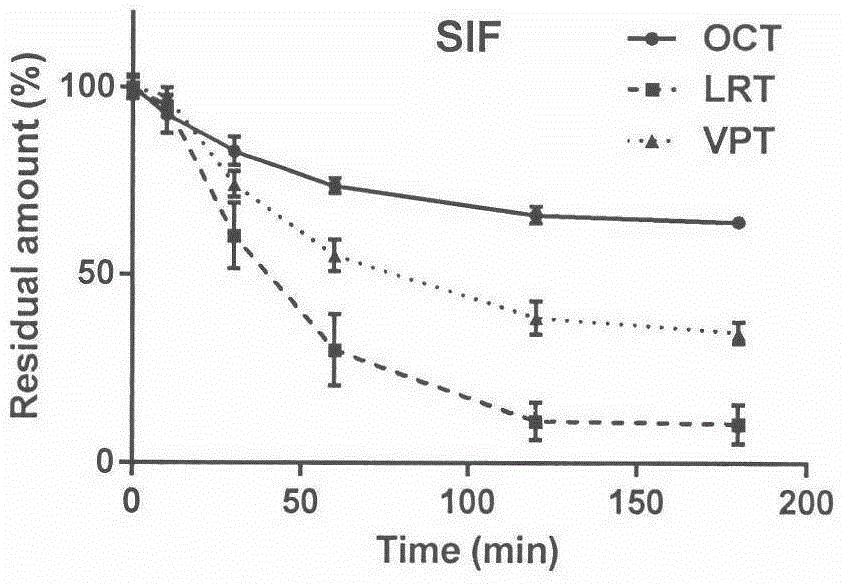
[0019] Example 2 Stability of three kinds of somatostatin analog polypeptides octreotide, vapreotide, and lanreotide in artificial intestinal juice:
[0020] Step 1: Prepare artificial intestinal juice according to the formula of the United States Pharmacopoeia. The specific formula is as follows: in 1000mL artificial intestinal juice, trypsin 10.0g, KH 2 PO 4 6.8g, adjust the pH value to 7.5±0.1 with NaOH, and incubate at 37°C for 30 minutes before use.
[0021] The second step: use the artificial intestinal juice in the first step to prepare 1 mL of octreotide, vapreotide, and lanreotide solutions with a concentration of 100 μg / mL, and make 3 copies in parallel, and incubate at 37°C for a certain period of time (0, 10, 30, After 60, 120, and 180 minutes), the reaction was terminated with 3 mL of glacial acetonitrile, centrifuged at 30,000 g for 10 minutes, and the supernatant was taken for detection by LC-MS / MS.
[0022] Step 3: Calculate the residual amount. Taking the ...
Embodiment 3
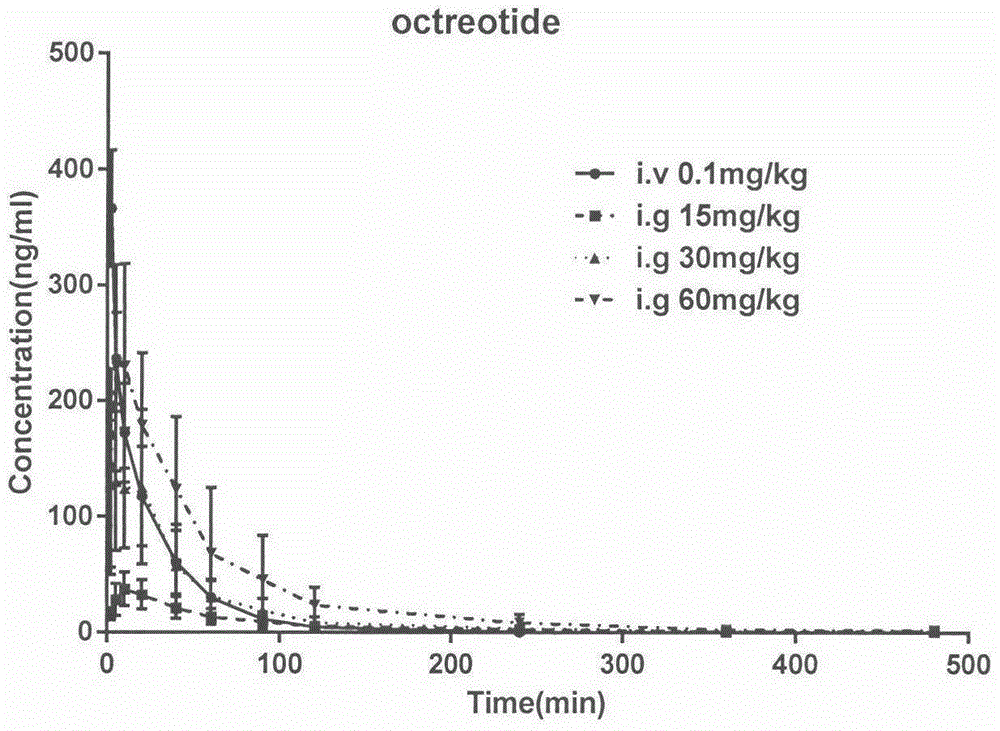
[0023] Example 3 Blood drug concentration over time after intravenous injection and intragastric administration of three somatostatin analogue polypeptides in rats:
[0024] The first step: 30 male SD rats, 180-200g, were randomly divided into 6 groups, 5 rats in each group, respectively octreotide intravenous injection group, octreotide intragastric administration group, vapreotide intravenous injection group, vapreotide infusion group Stomach group, lanreotide intravenous injection group, lanreotide intragastric administration group.
[0025] Step 2: Before administration, all rats were fasted without water for 12 hours. In the intravenous injection group of the three polypeptides, the rats were injected into the tail vein at a dose of 0.1 mg / kg, and blood was collected in heparinized EP tubes at 2, 5, 10, 20, 40, 60, 90, 120, and 240 minutes; In the gavage group, the dosage of 15, 30, and 60 mg / kg was gavaged with the aqueous solution of the three polypeptides, and the blo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com