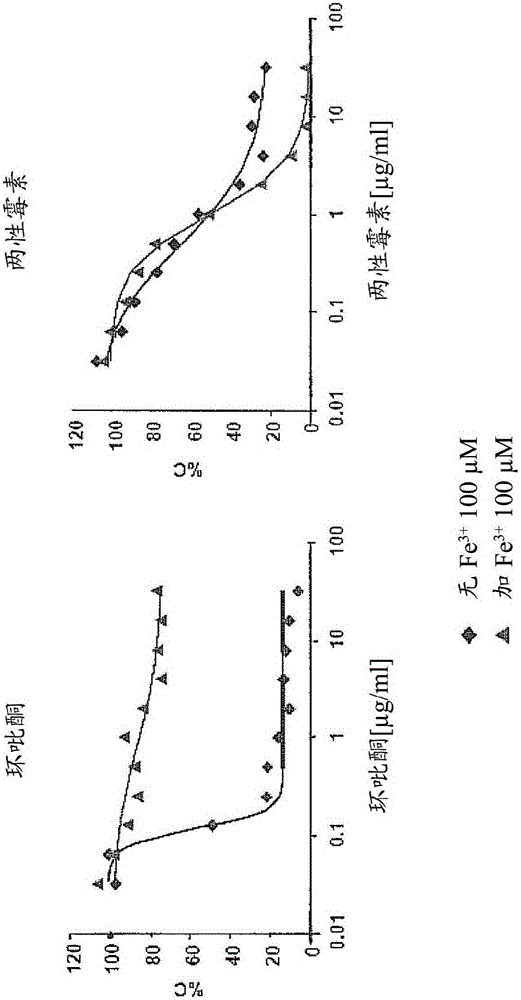
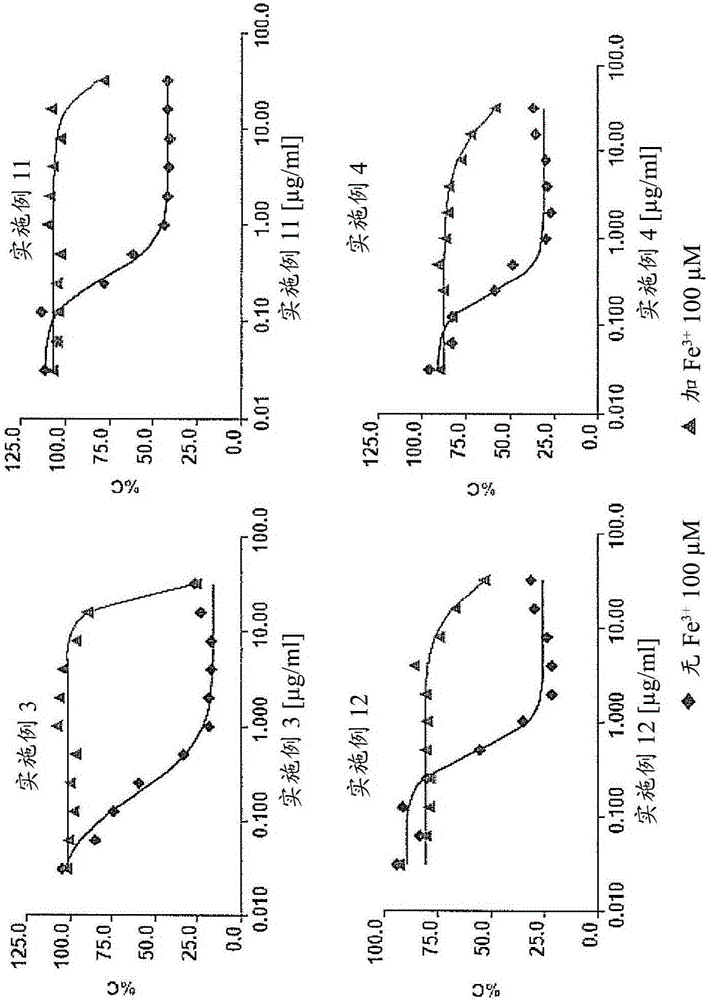
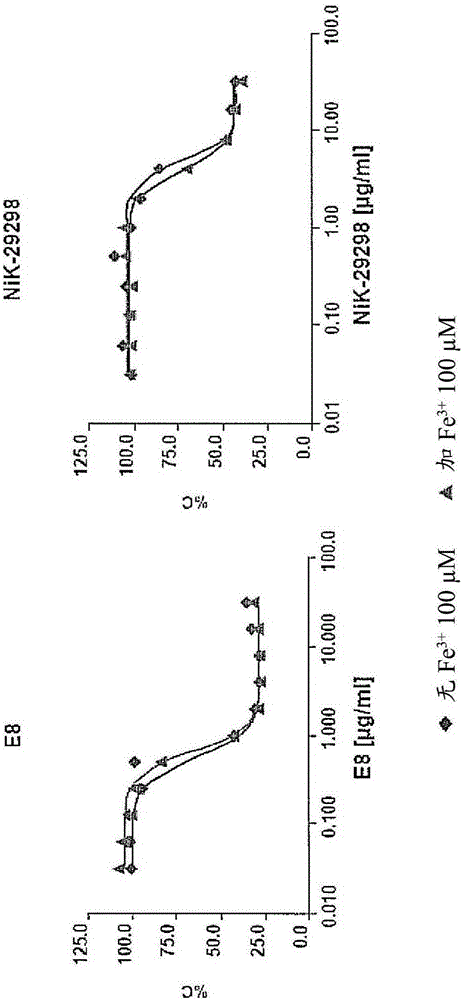
Secondary 8-hydroxyquinoline-7-carboxamide derivatives for use as antifungal agents
一种抗真菌剂、羧酰胺的技术,应用在用作抗真菌剂的仲8-羟基喹啉-7-羧酰胺衍生物领域,能够解决窄作用范围、药物不令人满意、药代动力学特性严重等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0289]
[0290] 8-Hydroxy-N-(thiophen-2-ylmethyl)quinoline-7-carboxamide
[0291] A mixture of 8-hydroxyquinoline-7-carboxylic acid (100 mg, 0.53 mmol) and bis(1H-imidazol-1-yl)methanone (86 mg, 0.53 mmol) in THF (8 mL) was heated to 45 °C under nitrogen. ℃ overnight. The reaction mixture was cooled to room temperature and thiophen-2-ylmethylamine (30 mg, 0.26 mmol) was added. The resulting mixture was stirred at room temperature for 24 hours. Additional thiophen-2-ylmethylamine (15 mg, 0.13 mmol) was added and the reaction mixture was stirred at room temperature for 60 hours. with H 2 O quenched the reaction mixture and evaporated THF to dryness. A saturated solution of sodium bicarbonate was added to the aqueous phase and extracted twice with DCM. by Na 2 SO 4 The separated organics were dried, filtered and concentrated under reduced pressure. The residue was purified by SPE-SI cartridge (2 g, DCM to DCM:MeOH 99:1 ) to provide the title compound (40 mg, 0.14 mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com