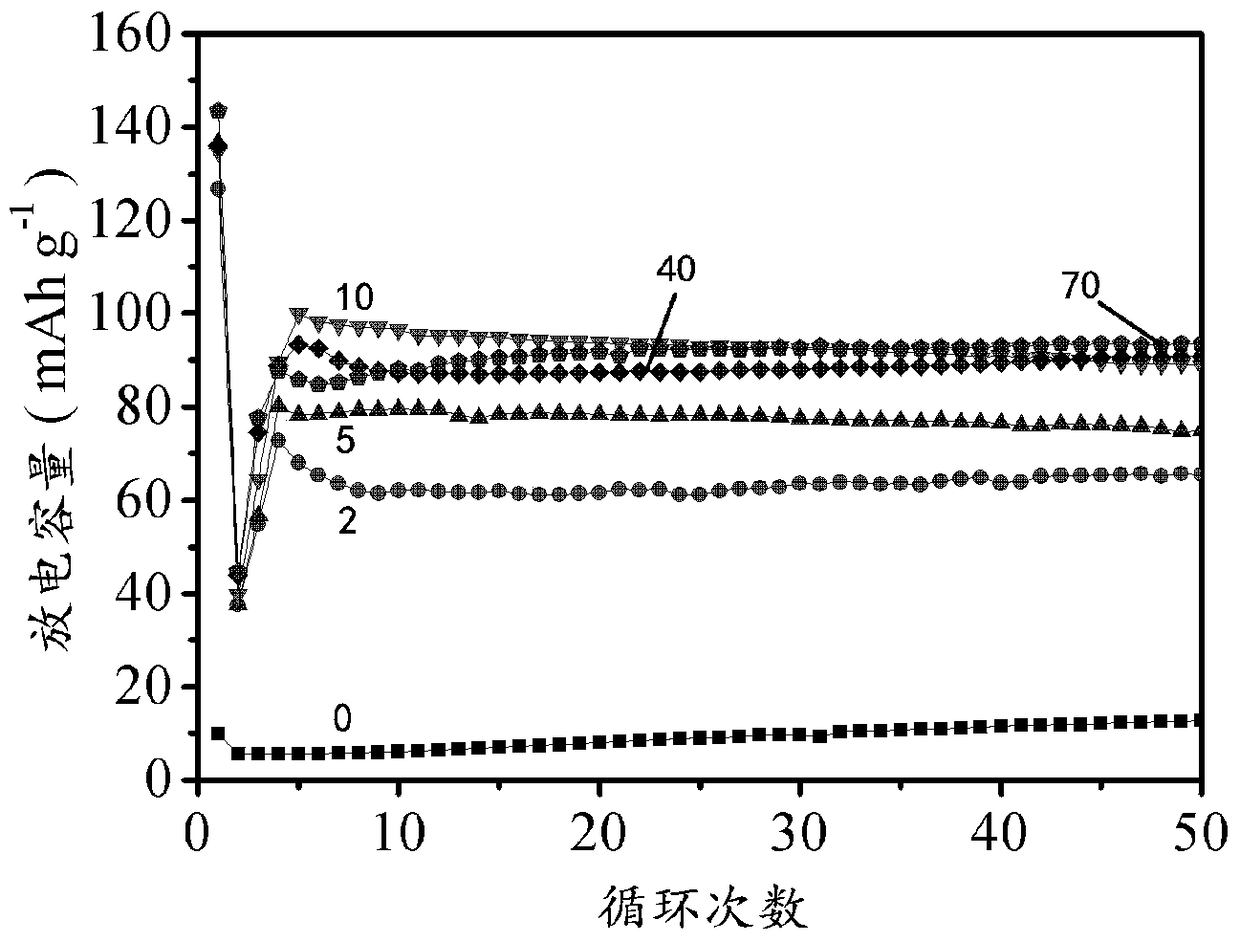
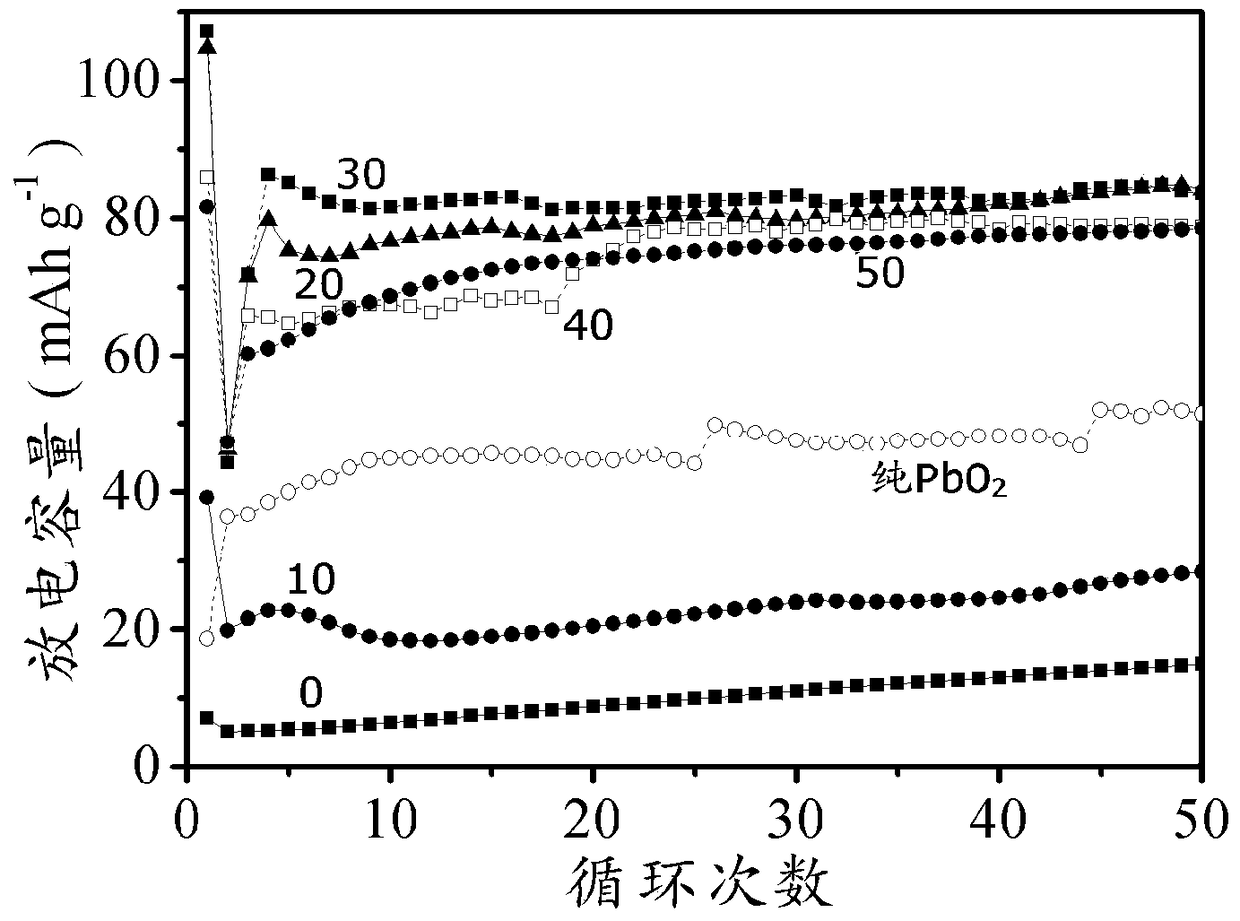
A positive electrode of a lead-acid battery using lead sulfate as an active material and a method for preparing a lead-acid battery using the positive electrode
A lead-acid battery and active material technology, applied in the direction of lead-acid battery electrodes, battery electrodes, circuits, etc., can solve the problems of unreported electrode cycle performance, low discharge specific capacity, poor plate performance, etc., to improve electrochemical performance , Pollution risk reduction, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] (1) Preparation of lead sulfate: the lead sulfate used can be prepared from the positive electrode material of the waste lead-acid battery, or prepared from lead salt and sulfuric acid or sulfate. The present invention only considers the former. The method is: for the positive electrode material of waste lead-acid battery, because it contains PbO 2 and PbSO 4 , first need to put PbO 2 reduction followed by desulfurization to give PbCO 3 or basic lead carbonate. For example, we have reported a method for the thermal reduction of waste lead-acid battery cathode materials in a closed container at 140 °C:
[0023] 3PbO 2 +CH 3 OH=2PbO+PbCO 3 +2H 2 O (1)
[0024] Combine the prepared PbO with H 2 SO 4 Reaction to get PbSO 4 :
[0025] PbO+H 2 SO 4 =PbSO 4 +H 2 O (2)
[0026] We found that the prepared PbSO 4 It is a good negative active material, but its performance is poor when used directly as a positive active material. However, experiments have found th...
Embodiment 1
[0045] (1) Gently disassemble the lead-acid battery to obtain the positive plate, and then separate the lead alloy grid from the electrode powder. The electrode powder is the positive electrode material of the waste lead-acid battery. Analysis of which PbSO 4 and PbO 2 content.
[0046] (2) Put the waste lead-acid battery positive electrode material into a stirring reactor, and then press the PbSO 4 Add sufficient amount of alkali solution, stir to make it react, and the reaction time is within 2h. The base can be any soluble carbonate or hydroxide (including ammonia). The solid was filtered and washed to remove soluble salts.
[0047] (3) Roast the solid obtained in the second step in air at 450°C for 2 hours to obtain Pb 3 o 4 .
[0048] (4) React soluble lead salt solution, such as lead nitrate, lead acetate or lead chloride, with sodium sulfate solution in a counterflow reactor to obtain ultrafine PbSO 4 .
[0049] (5) combine the lead sulfate that makes and the ...
Embodiment 2
[0052] Step is basically the same as Example 1, difference is: in step (3), solid is roasted 3h in 350 ℃ of air; In step (5), acetylene black is 0.1% of the quality of lead sulfate, and short fiber is the quality of lead sulfate 0.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com