A sea anemone cytolysin gigantoxin-4 monoclonal antibody and its preparation and application
A monoclonal antibody and cytolysin technology, which is applied in the fields of antibodies, extracellular fluid diseases, biochemical equipment and methods, etc., and can solve the problems that there is no Gigantoxin-4 antibody for sea anemone cytolysin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
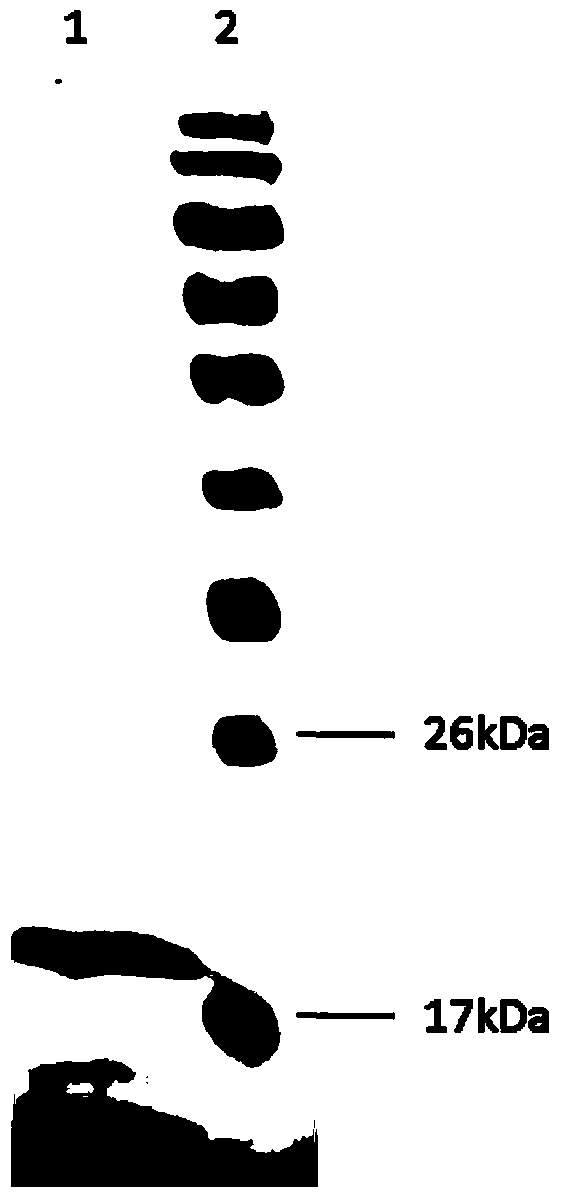
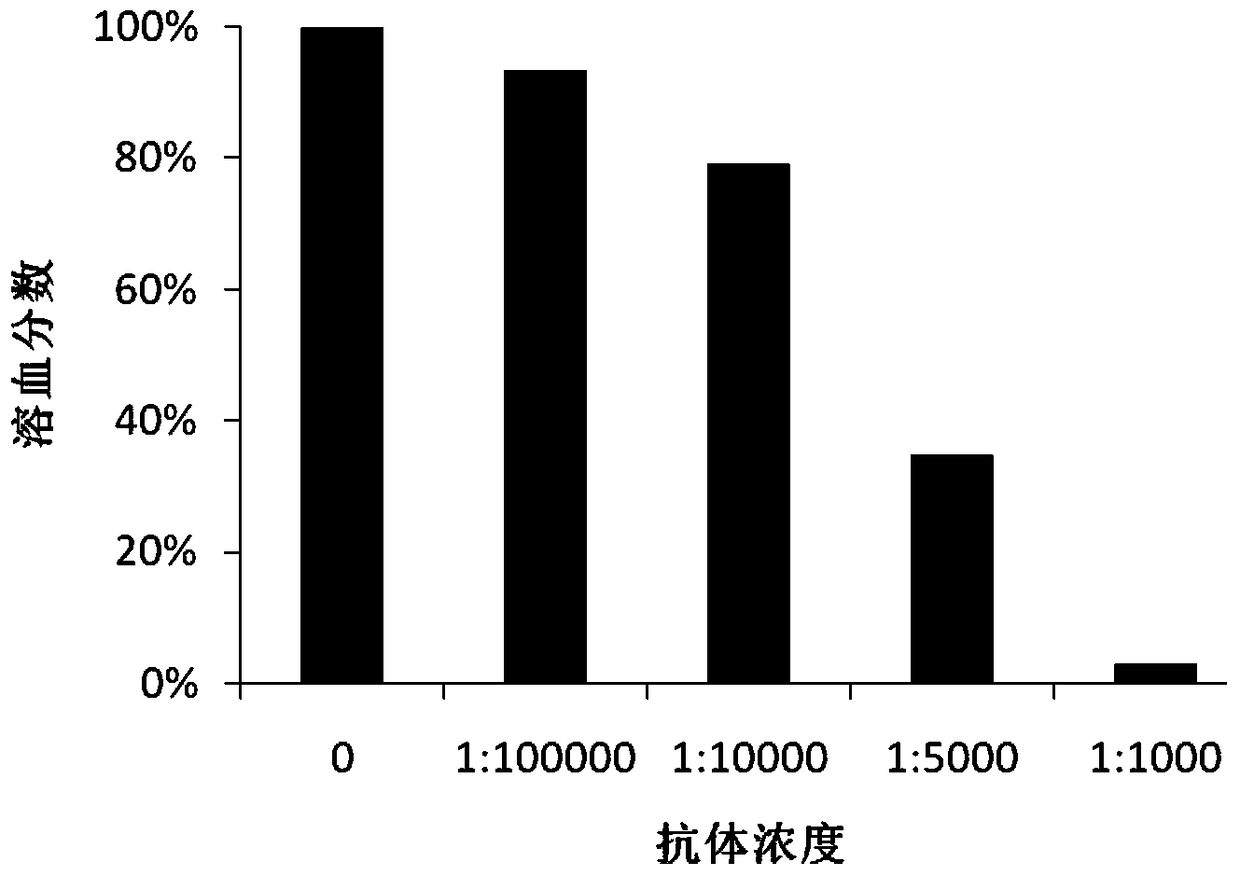
Image
Examples
Embodiment 1
[0029] Embodiment 1. The polypeptide sequence selection of anti-GT-4 monoclonal antibody and the preparation of polypeptide
[0030] Based on the analysis of the active site and transmembrane structure of GT-4, SEQ ID NO: 1 was selected as the epitope, and Abimate Biopharmaceutical (Shanghai) Co., Ltd. was entrusted to synthesize the polypeptide. The sequence is as follows:
[0031] Asp Val Ile Leu Pro Glu Phe Val Pro Asn Asn Lys (SEQ ID NO: 1)
[0032] Abimate Biomedical (Shanghai) Co., Ltd. was entrusted to synthesize the polypeptide, link the above polypeptide to the corresponding carrier, prepare and purify the antigen for subsequent immune response.
Embodiment 2
[0033] Example 2. Preparation and purification of anti-GT-4 monoclonal antibody
[0034] The polypeptide antigen purified in Example 1 was used for immunization, and 3 female BALB / c mice aged 8-12 weeks were immunized, and the mice were rapidly immunized. The initial immunization was performed subcutaneously under the skin around the shoulders and then double The legs were injected intramuscularly with approximately 1 / 8 of the immunogen per area and 1 / 2 of the remaining immunogen intraperitoneally. One week later, booster immunization with 50 μg, complete Freund's adjuvant, intraperitoneal injection. Two weeks later, booster immunization with 50 μg, incomplete Freund's adjuvant (IFA), intraperitoneal injection. For 3 weeks and later, 50 μg intraperitoneal injection was administered directly every week for booster immunization.
[0035] The spleen cells of the mouse with the best ELISA detection effect were fused with mouse myeloma cell SP2 / 0 cells (purchased from ATCC) at a ...
Embodiment 3
[0039] Example 3. Identification of Anti-GT-4 Monoclonal Antibodies
[0040] 1. Potency identification
[0041]Polypeptide antigen (4μg / ml) was coated on the ELISA plate, and the purified anti-GT-4 monoclonal antibody was carried out at 1:6.25K, 1:12.5K, 1:25K, 1:50K, 1:100K, 1:200K Dilute and add to the wells of the microtiter plate, select HIS antibody (HIS antibody 1mg / ml1:8K diluted with milk) as a positive control for system operation, and 5% milk as a negative control. After the reaction, goat anti-mouse IgG-HRP was added to develop the color. Positive judgment standard: the ratio (P / N) of the OD450 of the test well of the sample to the OD450 of the negative control well is ≥ 2.1, and the result shows that the antibody titer is 200K (Table 1).
[0042] Table 1 Antibody titer identification results
[0043] 6.25K
12.5K
25K
50K
100K
200K
Negative
Positive
3.205
2.711
2.364
1.475
0.954
0.607 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com