Preparation method of piperaquine derivative
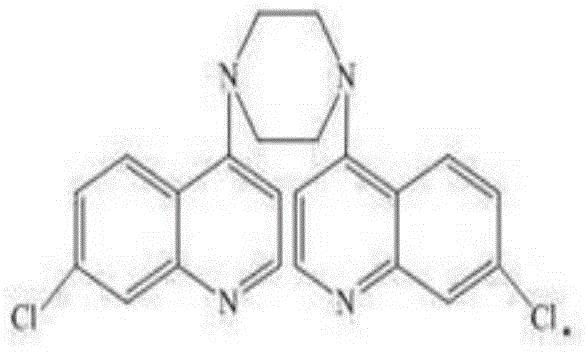
A technology of derivatives and piperaquine, which is applied in the preparation of 1,4-dipiperazine and the field of piperaquine derivatives Ⅲ, which can solve the problems that the synthesis and purification methods of piperaquine derivatives Ⅲ have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
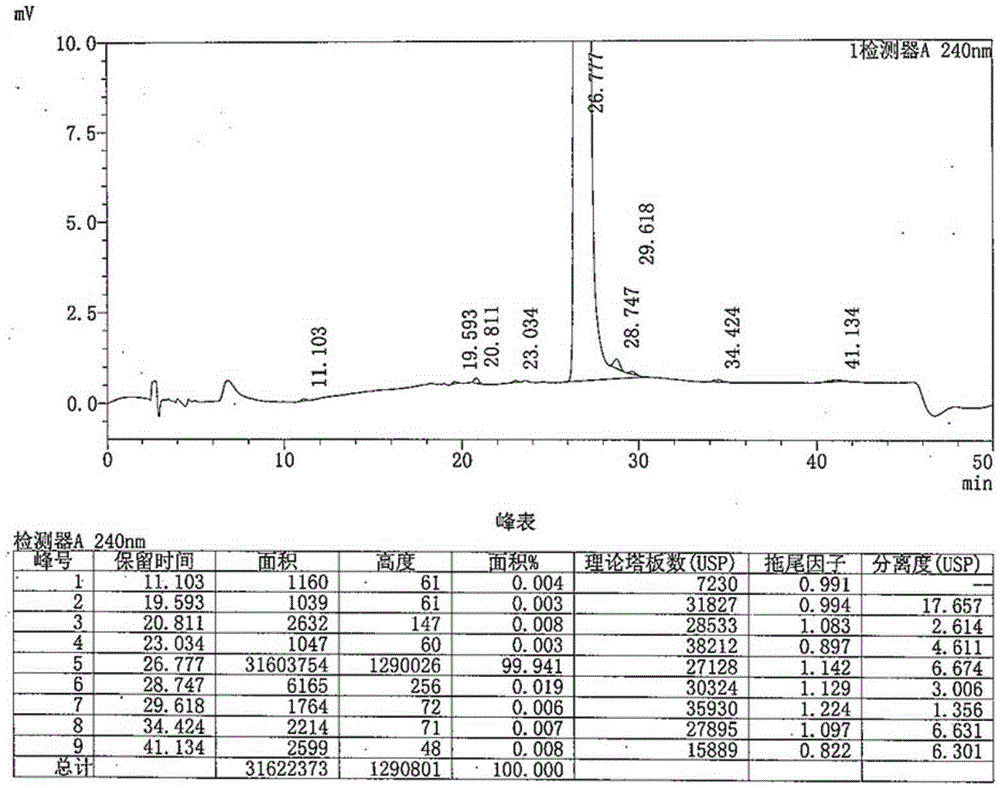
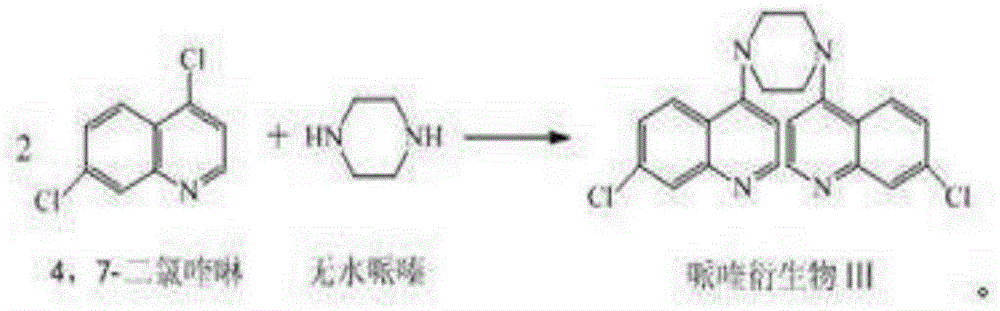
[0033] The preparation of embodiment 1 piperaquine derivative III
[0034] Add 100ml of ethanol, 39.6g of 4,7-dichloroquinoline, and 4.3g of anhydrous piperazine into the reaction flask, start stirring, and raise the temperature to reflux for 8 hours. After the reaction, distill out ethanol, add 200ml of 5% dilute hydrochloric acid solution, Stir for 30 minutes, cool to 20-25°C, and separate the solid by filtration. Transfer the filter cake into a reaction flask, add 100ml of 5% sodium hydroxide solution, stir at 20-25°C for 1 hour, filter and separate the solid; transfer the filter cake into a reaction flask, add 100ml of 5% dilute hydrochloric acid solution, Stir for 1 hour, filter and separate the solid; then use 5% sodium hydroxide solution and 5% dilute hydrochloric acid solution to beat for 3 times; transfer the separated solid into a reaction flask, add 200ml of purified water, and stir for 1 hour at 20-25°C , the solid was separated by filtration, and dried under redu...
Embodiment 2
[0035] The preparation of embodiment 2 piperaquine derivative III
[0036] Add 100ml of methanol, 59.6g of 4,7-dichloroquinoline, and 4.3g of anhydrous piperazine into the reaction flask, start stirring, and raise the temperature to reflux for 8 hours. After the reaction, distill off methanol, add 200ml of 5% dilute hydrochloric acid solution, Stir for 30 minutes, cool to 20-25°C, and separate the solid by filtration. Transfer the filter cake into a reaction flask, add 100ml of 5% sodium hydroxide solution, stir at 20-25°C for 1 hour, filter and separate the solid; transfer the filter cake into a reaction flask, add 100ml of 5% dilute hydrochloric acid solution, Stir for 1 hour, filter and separate the solid; then use 5% sodium hydroxide solution and 5% dilute hydrochloric acid solution to beat for 3 times; transfer the separated solid into a reaction flask, add 200ml of purified water, and stir for 1 hour at 20-25°C , the solid was separated by filtration, and dried under re...
Embodiment 3
[0037] The preparation of embodiment 3 piperaquine derivative III
[0038] Add 100ml of methanol, 39.6g of 4,7-dichloroquinoline, and 4.3g of anhydrous piperazine into the reaction flask, start stirring, and raise the temperature to reflux for 8 hours. After the reaction, distill off methanol, add 200ml of 5% dilute hydrochloric acid solution, Stir for 30 minutes, cool to 20-25°C, and separate the solid by filtration. Transfer the filter cake into a reaction flask, add 100ml of 5% sodium hydroxide solution, stir at 20-25°C for 1 hour, filter and separate the solid; transfer the filter cake into a reaction flask, add 100ml of 5% dilute hydrochloric acid solution, Stir for 1 hour, filter and separate the solid; then use 5% sodium hydroxide solution and 5% dilute hydrochloric acid solution to beat for 3 times; transfer the separated solid into a reaction flask, add 200ml of purified water, and stir for 1 hour at 20-25°C , the solid was separated by filtration, and dried under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com