Topical treatment of localized scleroderma
A scleroderma, topical application technology, applied in the direction of skin diseases, allergic diseases, organic active ingredients, etc., can solve the problems that have not shown continuous benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1 - Imatinib mesylate formulation for topical application
[0124] background
[0125] The active ingredient, imatinib mesylate (IM), is well known and known to be safe and effective as a systemic chemotherapeutic agent. IM is approved by the U.S. Food and Drug Administration (FDA) and is sold under the trade name. IM can be used to treat chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GISTs) and other malignancies. IM acts as a selective inhibitor of BCR-ABL tyrosine kinase and has additional activity against α-PDGFR, β-PDGFR and KIT receptor kinases. To our knowledge, no studies have examined the pharmacodynamic effects of topically administered IM. It is an object of the present invention to develop topical formulations for the treatment of the connective tissue disease systemic sclerosis (SSc) or scleroderma.
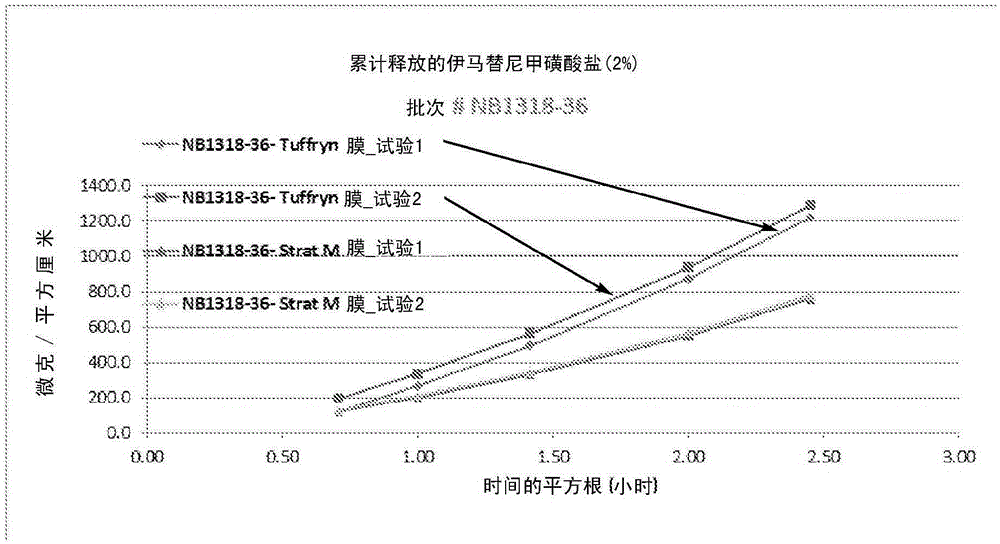
[0126] brief description
[0127] The target IM concentration (20%) was found to be achieved in 3 out of 20+ solvents (incl...
Embodiment 2
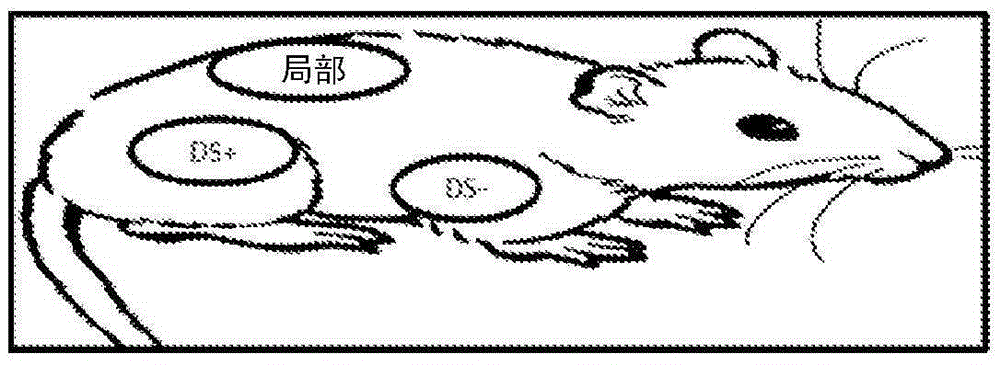
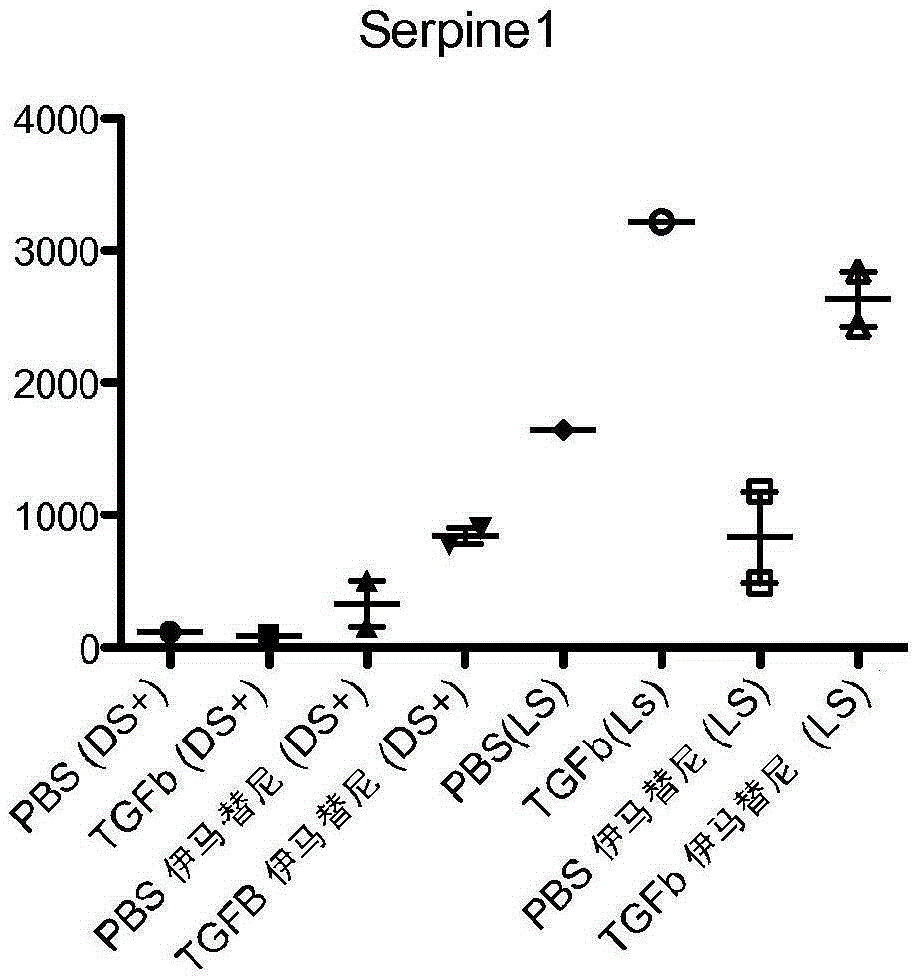
[0185] Example 2 - Animal Studies
[0186] C57BL / 6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). An osmotic pump (Alzet, model 2001) designed to deliver 1 μL / h was loaded with TGF-β (1.25 μg) in phosphate buffered saline (PBS) supplemented with 0.1 mg / mL bovine serum albumin (BSA). Pumps were implanted subcutaneously in 8-week-old mice, which were subsequently treated with imatinib or placebo cream. After 7 days, the mice were sacrificed and the skin around the pump outlet (~1 cm 2 ) to prepare RNA, or fix in formalin. Skin from mice treated with PBS and TGF-β was analyzed using nanostring technology. A panel of 50 genes including inflammatory genes, macrophage markers, TGF-β regulatory genes, etc. was analyzed. 100 ng RNA per sample was used and gene expression was normalized to the expression of 8 housekeeping genes.
[0187] See, eg, Christmann, R.B. et al., Arthritis Rheum. 2013 May;65(5):1335-46 (teaching that thymic stromal lymphopoietin is upregula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com