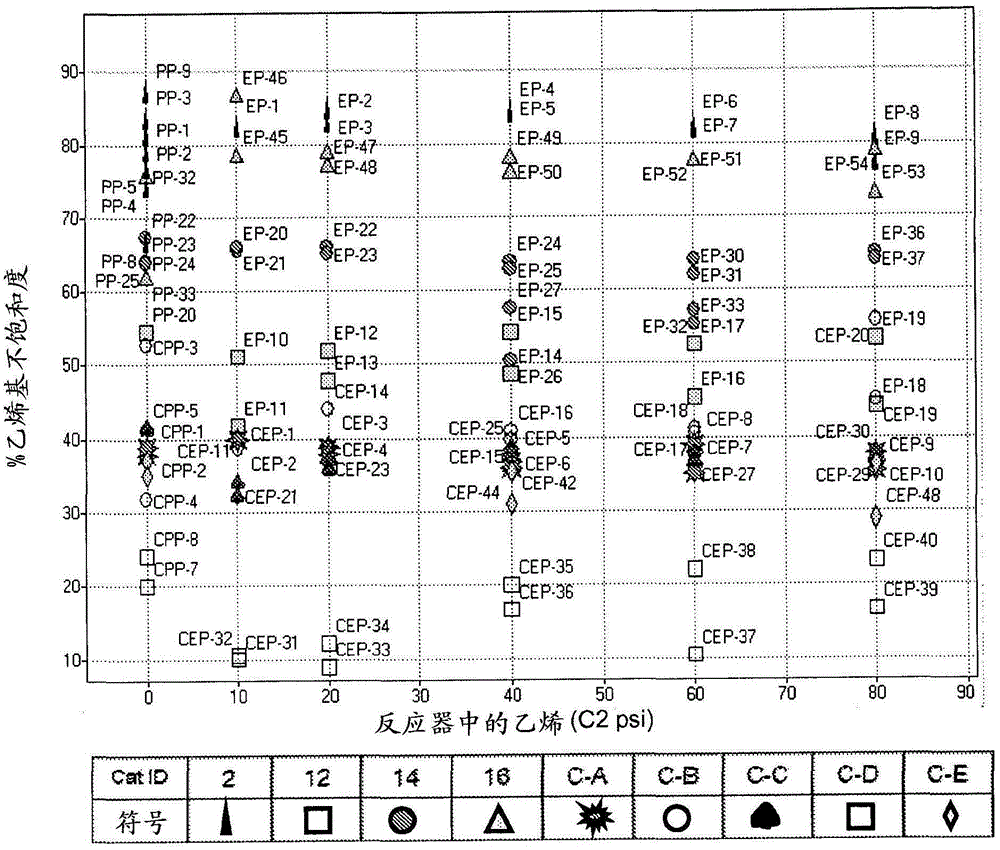
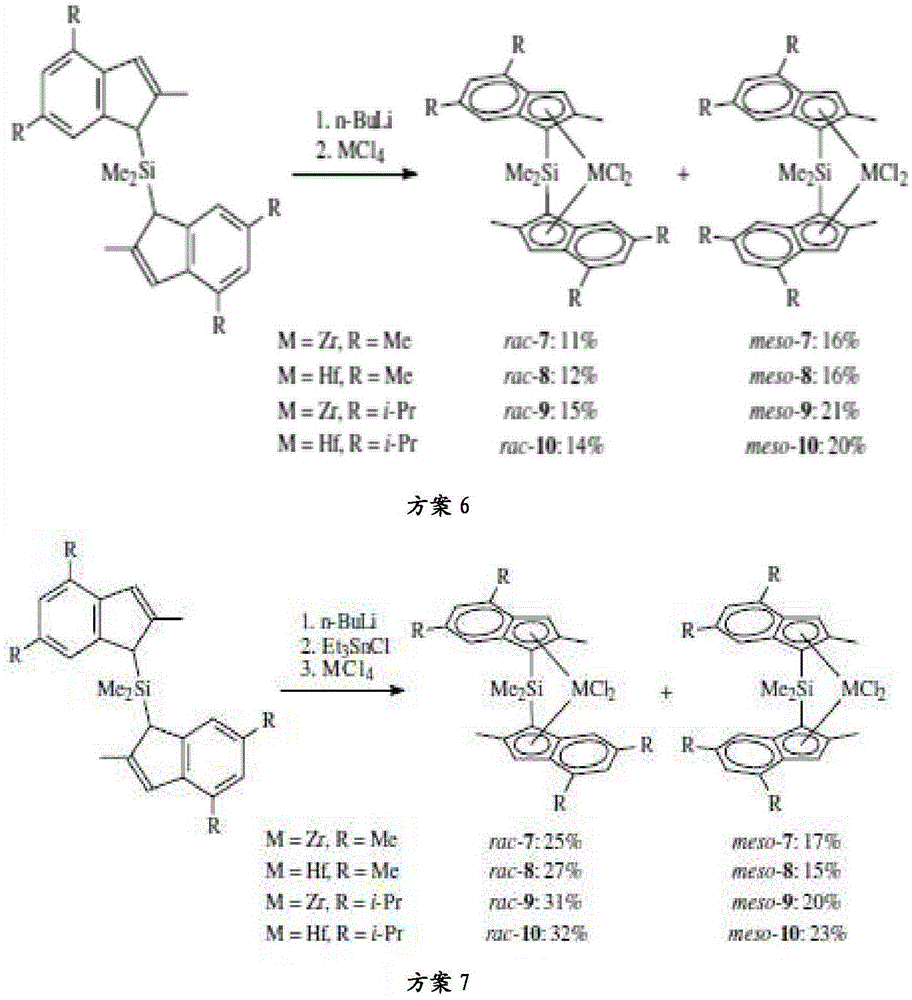
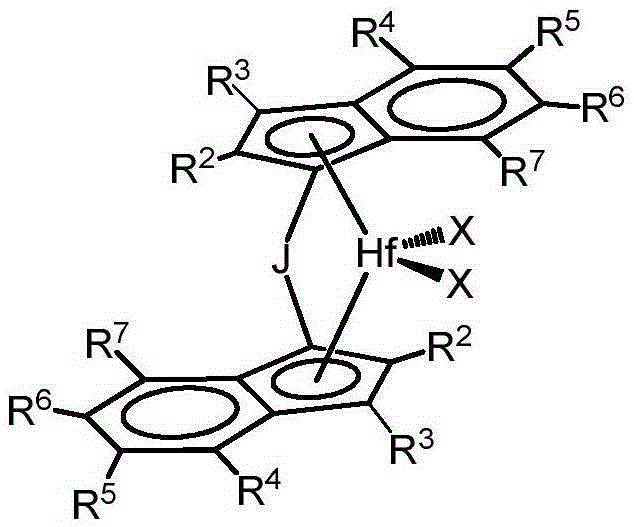
Substituted metallocene catalysts
A metallocene catalyst and catalyst technology, applied in the direction of metallocene, organic chemistry, chemical instruments and methods, etc., can solve the problem of no cyclic bridging group
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0400] Example 1: Synthesis of 1,1-silacyclopentanediyl-bis(2,4,7-trimethylinden-1-yl) Hafnium dichloride, or cyclotetramethylenesilylene-bis(2,4,7-trimethylinden-1-yl) Hafnium dichloride(1)
[0401] 1,1-bis(2,4,7-trimethyl-1H-inden-1-yl)silacyclopentane
[0402]
[0403] To a solution of 39.6g (0.25mol) 2,4,7-trimethylindene in 350ml of ether, slowly add 2.5M in 100ml (0.25mol) of hexane n BuLi. The resulting suspension was stirred at room temperature for 30 minutes, then 50 ml THF was added to dissolve the precipitate. The resulting mixture was stirred for 1 h, then cooled to -60 °C and 1.12 g (12.5 mmol) CuCN was added. The resulting mixture was stirred at room temperature for 40 minutes, then 19.4 g (0.125 mmol) of 1,1-dichlorosilacyclopentane were added in one portion. The mixture was stirred overnight at room temperature, then 200 ml of cold water were added. Separate the organic layer. The aqueous layer was extracted with 2x50ml dichloromethane. The com...
Embodiment 2
[0407] Example 2: Synthesis of racemic-1,1-silacyclopentanediyl-bis(2,4,7-trimethylindene -1-yl) hafnium dimethyl, also known as rac-cyclotetramethylenesilylene-bis(2,4,7- Trimethylinden-1-yl) hafnium dimethyl(2)
[0408]
[0409] To a suspension of 18.2 g (28.2 mmol) of the mixture of rac-, meso-hafnocene dichloride and LiCl (1, from Example 1) obtained above in 250 ml THF was added 35 ml (107 mmol) in ether 3.05MMeMgBr. The mixture was stirred overnight at 60°C, then evaporated to dryness. The residue was treated with 250 ml of hot toluene, which was then stripped off in vacuo. The product formed was extracted from the residue using 300 ml of hot methylcyclohexane. Crystals precipitated from the extract at -30°C were collected, and the mother liquor was used again to extract the product. This procedure was repeated until no solid material precipitated from the extract at -30°C. The combined precipitates were washed with 2x40ml THF and dried in vacuo. This proce...
Embodiment 3
[0410] Example 3: Synthesis of racemic-1,1-silacyclopentanediyl-bis(2-ethyl-4-methyl Inden-1-yl) hafnium dichloride, also known as rac-cyclotetramethylenesilylene-bis(2-ethyl -4-Methylinden-1-yl) hafnium dichloride (3)
[0411] 4-Bromo-2-ethylinden-1-one
[0412]
[0413]To a solution of sodium ethoxide prepared from 30.4 g (1.32 mol) of sodium metal and 830 ml of dry ethanol was added 364 g (1.10 mol) of diethyl (2-bromobenzyl)malonate. The solution was stirred for 10 minutes, then 98.5 ml (1.32 mol) of bromoethane were added portionwise with vigorous stirring for 15 minutes. The obtained mixture was refluxed for 4 h, then a solution of 185 g (3.30 mol) KOH in 450 ml water was added. The mixture was refluxed for 4 h, then ethanol was distilled off at atmospheric pressure. The resulting solution was extracted with diethyl ether, then the aqueous layer was acidified with 12M HCl to pH 1-2, then extracted with 3x300ml ethyl acetate. The combined organic extracts we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com