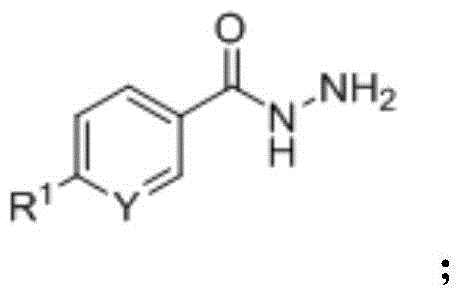
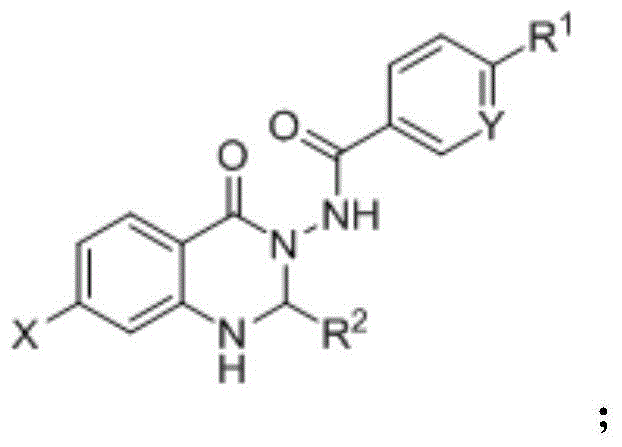
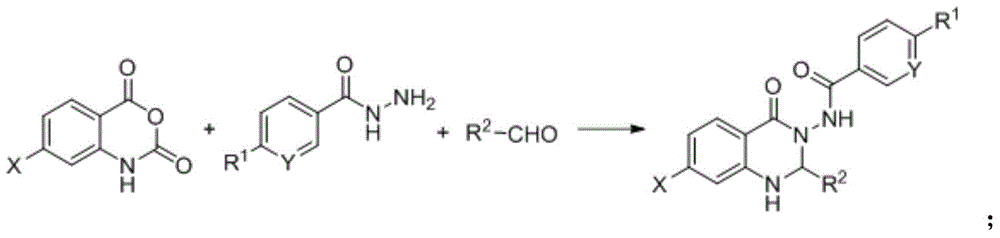
A kind of synthetic method of quinazolinone FPR2 formyl peptide receptor agonist
A formyl peptide receptor and quinazolinone technology, which is applied in the field of synthesis of quinazolinone FPR2 formyl peptide receptor agonists, can solve problems such as complicated preparation steps, simplify the preparation process and reduce the complexity of preparation The effect of high degree and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh isatoic anhydride, aromatic hydrazide compounds, and aromatic aldehyde compounds as raw materials according to the ratio of the amount of substances 1:1:1; mix the weighed raw materials in ethanol, use nano-copper oxide as a catalyst, and add catalyst The ratio of the amount of the isatoic anhydride to the amount of the substance of isatoic anhydride is 1:0.02, and a one-pot synthesis reaction is carried out, the reaction temperature is 60 ° C, and the reaction time is 6 hours; the reactant after the reaction is separated and purified by column chromatography. A quinazolinone FPR2 formyl peptide receptor agonist is prepared.
Embodiment 2
[0042] Weigh isatoic anhydride, aromatic hydrazide compounds, and aromatic aldehyde compounds as raw materials according to the ratio of the amount of substances 1:1:1; mix the weighed raw materials in ethanol, use nano-cerium oxide as a catalyst, and add catalyst The ratio of the amount to the amount of isatoic anhydride is 1:0.05, and the one-pot synthesis reaction is carried out, the reaction temperature is 70 ° C, and the reaction time is 8 hours; the reactant after the reaction is separated and purified by column chromatography. A quinazolinone FPR2 formyl peptide receptor agonist is prepared.
Embodiment 3
[0044] Weigh isatoic anhydride, aromatic hydrazide compounds, and aromatic aldehyde compounds as raw materials according to the ratio of the amount of substances 1:1:1; mix the weighed raw materials in ethanol, and use nanometer iron tetroxide as a catalyst, The ratio of the amount of catalyst added to the amount of isatoic anhydride is 1:0.08, a one-pot synthesis reaction is carried out, the reaction temperature is 90 ° C, and the reaction time is 10 h; the reactants after the reaction are separated by column chromatography Purify to obtain quinazolinone FPR2 formyl peptide receptor agonist.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com