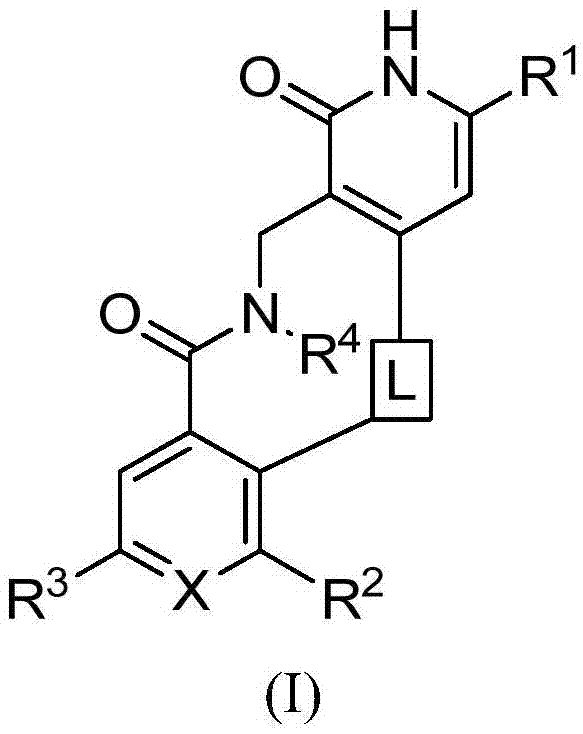
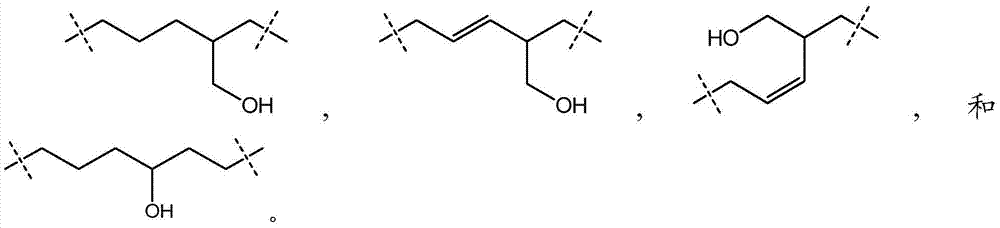
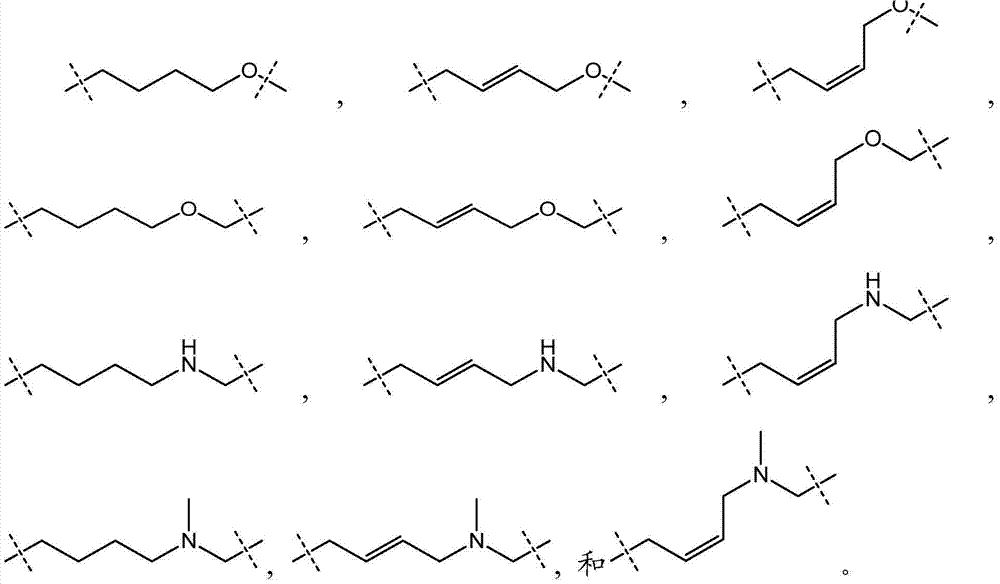
Enhancer of Zeste homolog 2 inhibitors
A compound, C2-C8 technology, applied in the field of compounds, can solve problems such as the loss of function of tumor suppressors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0436] Example 1: (E)-10-((trans-4-aminocyclohexyl)oxy)-12-chloro-3-methyl-5,6,15,16-tetrahydrobenzo[c]pyrido [4,3-j][1]Azacyclododecanentaene-1,14(2H,9H)-dione hydrochloride
[0437] (a) 4-(but-3-en-1-yl)-2-methoxy-6-methylnicotinonitrile
[0438]
[0439] To a solution of 2-methoxy-4,6-dimethylnicotinonitrile (1.5 g, 9.25 mmol) in THF (40 mL) was added LiHMDS (10.17 mL, 10.17 mmol) at -78°C, and the mixture was placed in - Stir at 78°C for 1 hour. 3-Bromoprop-1-ene (0.880 mL, 10.17 mmol) was added, and the mixture was stirred at -78°C for 1 hour and warmed to 0°C over 1 hour. The mixture was then stirred at 0°C for 3 hours. The reaction was saturated with NH 4 Quenched with aqueous Cl and extracted with EtOAc (3x). The combined organics were subjected to Na 2 SO 4 Dried and concentrated. The residue was subjected to reverse phase HPLC (using Trilution software, with phenomenonex Gemini5uC18(2) 100A, AXIA30x100mm5 micron, 10-minute run (30mL / min, 40% CH 3 CN / H 2 ...
Embodiment 2
[0477] Example 2: (E)-12-chloro-10-((trans-4-(dimethylamino)cyclohexyl)oxy)-3-methyl-5,6,15,16-tetrahydrobenzo [c]pyrido[4,3-j][1]azacyclododecapentaene-1,14(2H,9H)-dione
[0478]
[0479] To (E)-10-((trans-4-aminocyclohexyl)oxy)-12-chloro-3-methyl-5,6,15,16-tetrahydrobenzo[c]pyrido[4, 3-j][1]Azacyclododecenepentaene-1,14(2H,9H)-dione hydrochloride (230mg, 0.467mmol) in MeOH (8mL) slurry was added formaldehyde (0.278mL ,3.74mmol), NaBH 3 CN (147 mg, 2.335 mmol), then AcOH (0.027 mL, 0.467 mmol) was added. The resulting mixture was stirred overnight at room temperature. The reaction mixture was concentrated, and MeOH was added. The resulting suspension was filtered to give a residue and a filtrate containing both product. The residue was subjected to reverse phase HPLC ( Instrument, Trilution software, WatersSunFirePrepC18OBD5uM, 19x50mm column, using 10-50% CH containing 0.1% TFA 3 CN aqueous solution) purification. The resulting fractions were concentrated in vacuo ...
Embodiment 3
[0480] Example 3: 12-chloro-10-((trans-4-(dimethylamino)cyclohexyl)oxy)-3-methyl-6,7,8,9,15,16-hexahydrobenzo [c]pyrido[4,3-j][1]azacyclododecapentaene-1,14(2H,5H)-dione
[0481]
[0482] (E)-12-chloro-10-((trans-4-(dimethylamino)cyclohexyl)oxy)-3-methyl-5,6,15,16-tetrahydrobenzo[c] Pyrido[4,3-j][1]azacyclododecapentaene-1,14(2H,9H)-dione (26 mg, 0.054 mmol) in EtOAc (2 mL) and MeOH (10 mL) The solution was degassed with nitrogen for 5 min, then platinum (10 wt% on charcoal, 10 mg) was added and the solution was purged with nitrogen for an additional 5 min. The reaction mixture was stirred under a hydrogen atmosphere (balloon) for 8 hours. The reaction mixture was filtered and the filtrate was concentrated in vacuo to give 12-chloro-10-((trans-4-(dimethylamino)cyclohexyl)oxy)-3-methyl-6,7,8,9, 15,16-Hexahydrobenzo[c]pyrido[4,3-j][1]azacyclododecapentaene-1,14(2H,5H)-dione (20 mg, 77%), It is a white solid. LC-MS(ES) m / z=244(main product), 486[M+H] + (secondary product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com