renal progenitor cells
A progenitor cell and cell technology, applied in the field of kidney development, can solve problems such as lack of understanding and hindering the realization of human pluripotent stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
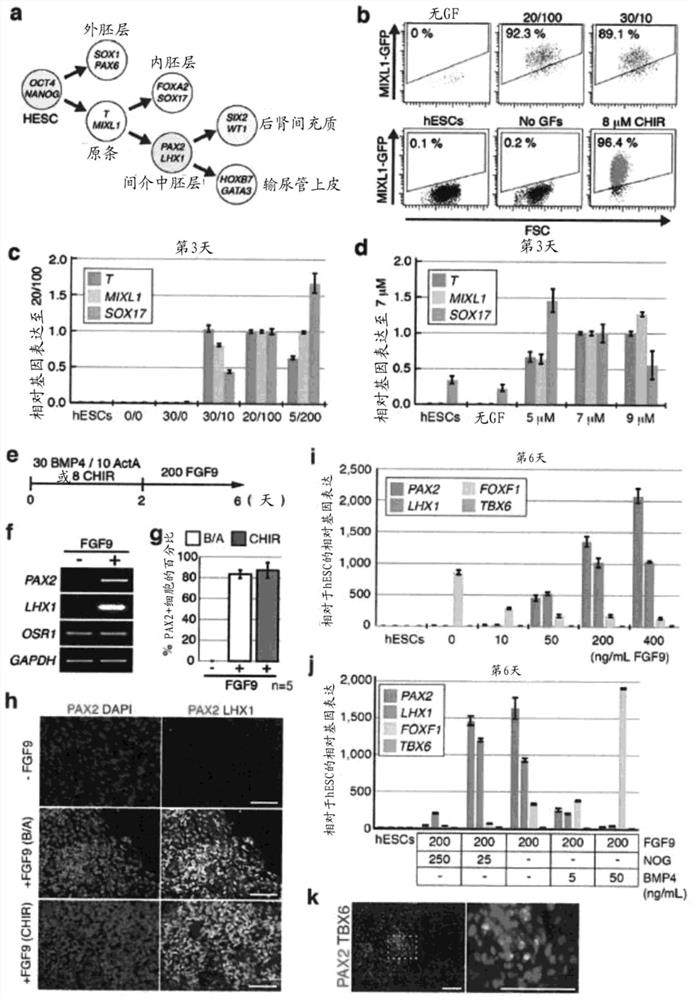
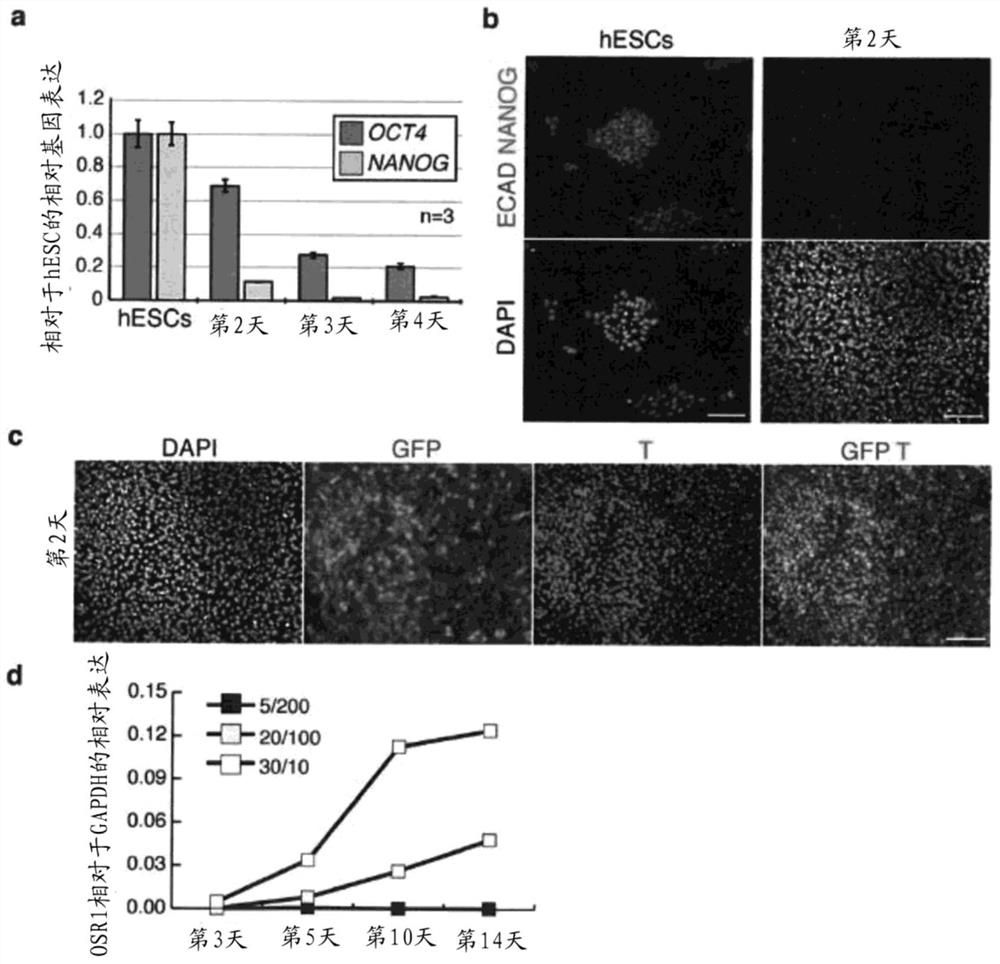
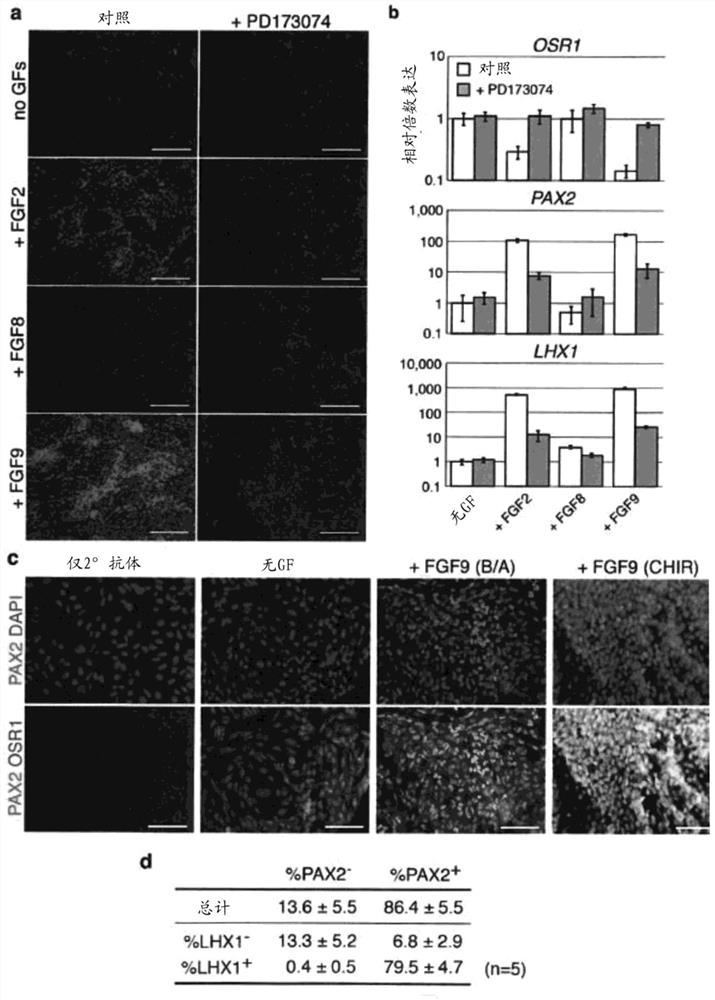
[0152] Materials and methods
[0153] hESC culture and differentiation
[0154] In supplemented with 20% KnockOut Serum Replacement (Life Technologies), 100 μM MEM NEAA (Life Technologies), 110 μM 2-Mercaptoethanol (Life Technologies), 1x Penicillin / Streptomycin (Life Technologies), 1x Glutamax (Life Technologies) and 10 ng / HES3 (MIXL1 GFP / wt )cell. The day before initiation of differentiation, cells were incubated at 12,000-15,000 cells / cm 2 Plate on Matrigel coated 96-well plates. After overnight culture, cells were exposed to 30ng / mL BMP4 (R&Dsystems) and 10ng / mL Activin A (R&Dsystems) or 8μM CHIR99021 in previously established serum-free medium APEL for 2-3 days, and then exposed to APEL culture 200 ng / mL FGF9 and 1 μg / mL heparin in the medium were maintained for 4 days to induce IM cells. In the case of BMP4 / Activin A induction, cells were subsequently exposed to 200 ng / mL FGF9, 50 ng / mL BMP7, 0.1 μM RA and 1 μg / mL heparin for 4-11 days. In the case of CHIR99021 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com