Targets for treating tumors
A technology for tumor treatment and targeting, which is applied in the field of tumor and chemotherapy, and can solve problems that have not been studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0229] Example 1 Synergistic inhibition of NF-κB activity
[0230] Overview: Typically, in vitro NF-κB activity is measured by artificial reporter gene assays, electronic gel retardation assays, and more recently DNA binding and ELISA methods. However, these methods all employ exogenous DNA oligonucleotides or constructs to measure DNA-binding transcriptional activity of specific NF-κB consensus and NF-κB promoter sequences. Furthermore, the NF-κB promoter function is different from the true promoter sequence. Real promoters require complex interactions between multiple protein molecules, while artificial NF-κB promoters may introduce artificial effects.
[0231] Experimental method: UM-SCC6 cells were transfected with effectene (Qiagen) 1) 20% dominant negative IKK1-KA (K44A) and 80% pUC19, 2) 20% dominant negative IKK2-KA (K44A ) and 80% pUC19 plasmid transfection, 3) 20% dominant negative IKK1-KA (K44A), 20% dominant negative IKK2-KA (K44A) and 60% pUC19, 4) 20% pcDNA3 an...
example 2
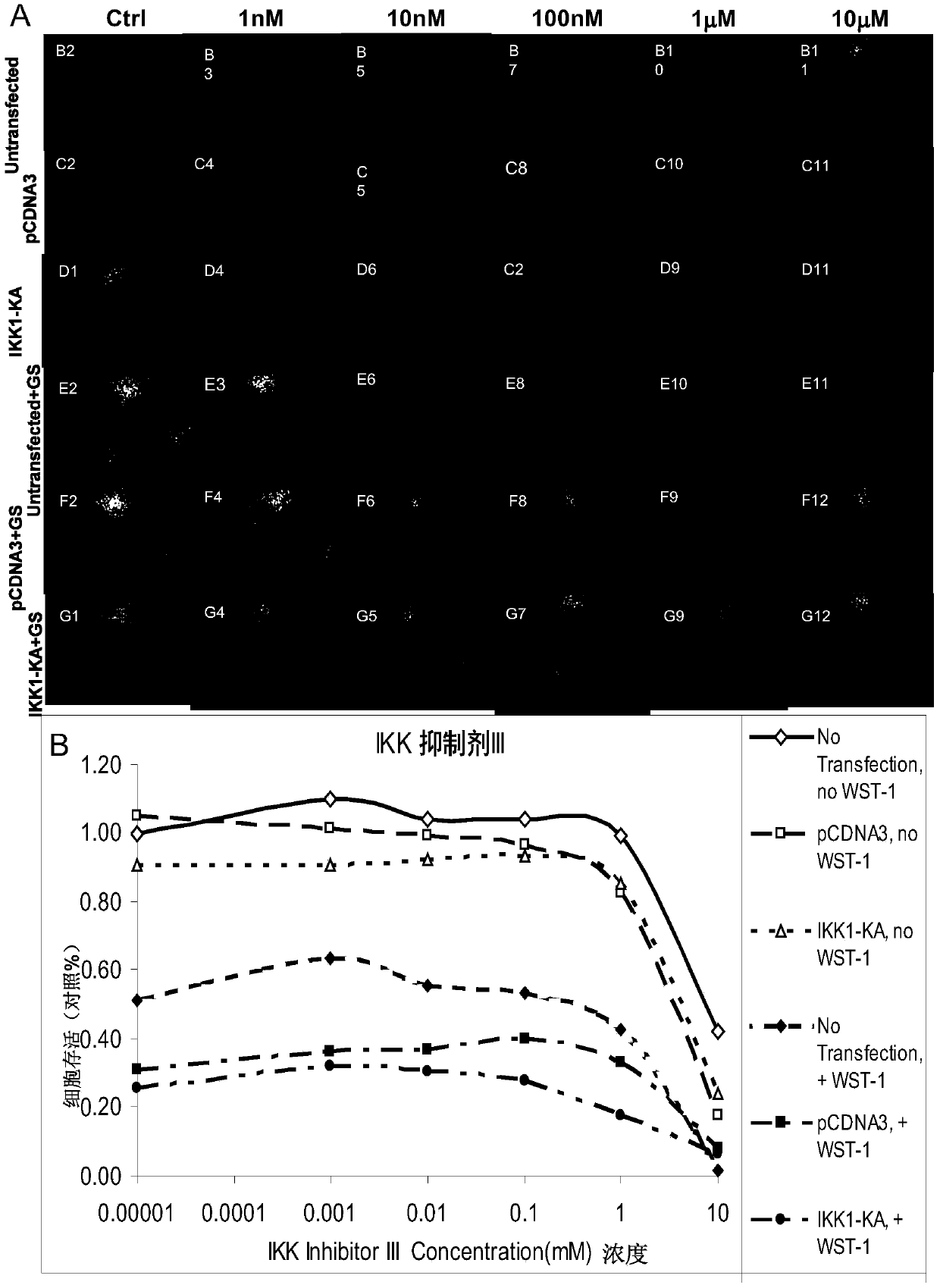
[0233] Example 2 Simultaneous inhibition of IKK1 and IKK2 can also lead to cancer cell death
[0234] In addition to inhibiting the activity of NF-κB, simultaneous transfection of SCC-6 cells with K44A-IKK1 and K44A-IKK2 also resulted in cell death ( figure 2 ). 48 hours after simultaneous transfection of K44A-IKK1andK44A-IKK2, the number of cells decreased by 85% ( figure 2 WST–1nono) and massive cell death ( figure 2 b). The data represent the average of 7 parallel experiments.
[0235] This result suggests that inhibition of NF-κB activity and resulting cancer cell death can only be achieved by simultaneously inhibiting both IKK1 and IKK2. In addition, co-transfected K44A-IKK1 and K44A-IKK2sensitize also enhanced the sensitivity of UM-SCC-6 cells to cisplatin and 5-FU by 10 to 100 times.
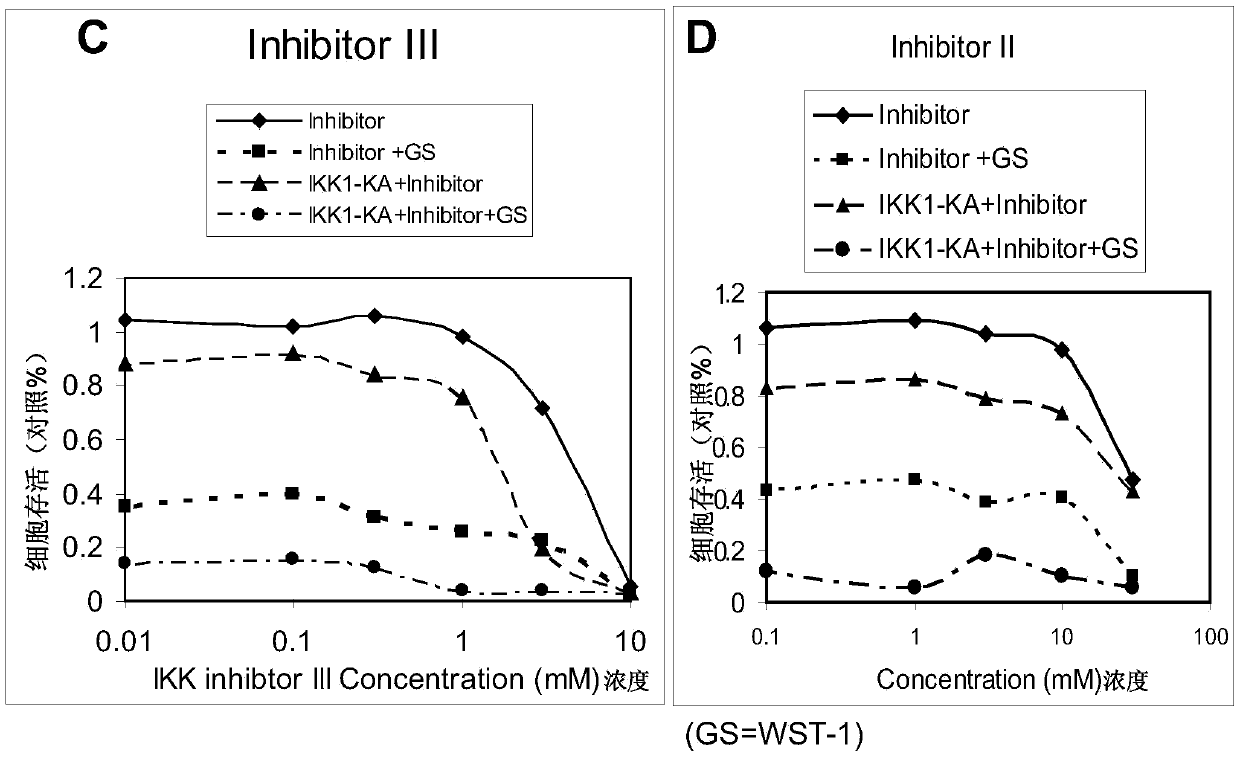
[0236] Example 2 Tetrazolium dye WST-1r increases cancer cell death caused by double inhibition of IKKs.
[0237] In this study, the conventional WST-1r method could not accurate...
example 4
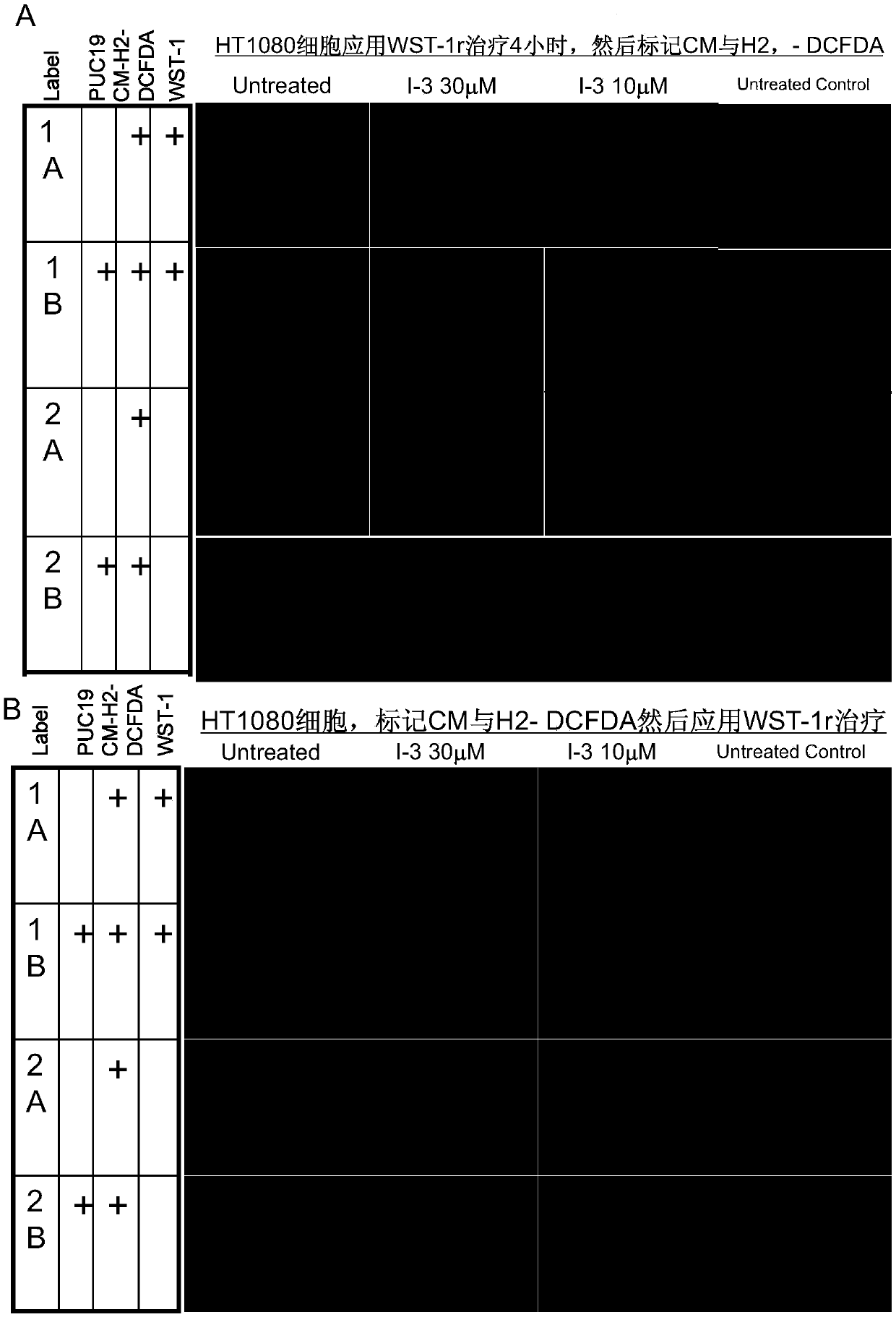
[0241] Example 4 WST-1 promotes the death of HT1080 human sarcoma cells induced by three combination treatments.
[0242]Methods: HT1080 cells were cultured in 96-well plates and transfected with pUC19, pCDNA3, IKK1-KA, IKK1-KA+PUC19, and pCDNA3+pUC19 DNA plasmids, and then sequentially applied IKK inhibitor and WST-1r. Except for the control group, each group of cells was transfected with one of the above-listed plasmids for 24 hours, and then applied 3-30 MIKK inhibitor III, 24 hours later, and then applied WST for 4 hours, and then continued to culture overnight and then carried out with CCK8 kit Measured and measured 24, 48 and 96 hours thereafter.
[0243] The data showed that (1) tumor cells were transfected with DNA and applied IKK inhibitor III, and treated with WST-1r 24, 48 and 96 hours after treatment, all exhibited IKK inhibitor III dose-dependent cell growth inhibition and reduced cell survive. However, there was no significant difference between transfection pU...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com