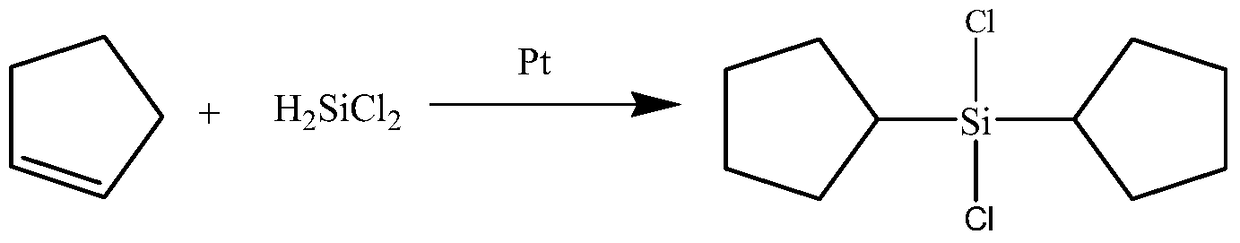
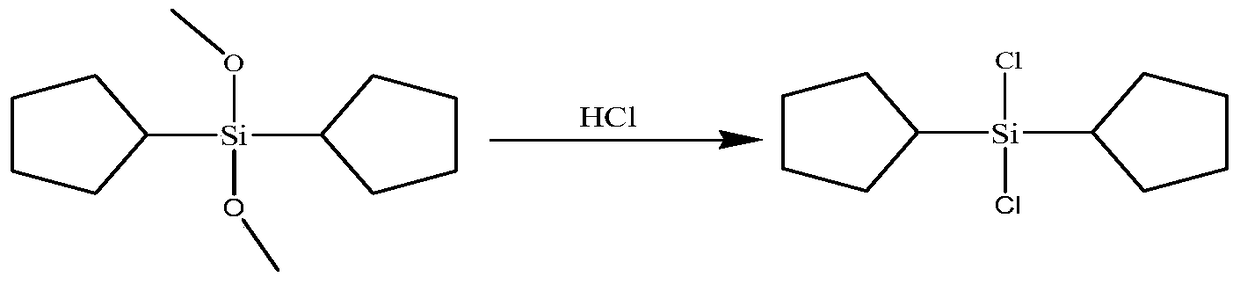
Synthesis process of dicyclopentyl dichlorosilane
A technology of dicyclopentyldichlorosilane and dicyclopentyldimethoxysilane, which is applied in the field of dicyclopentyldichlorosilane synthesis technology, can solve complex process, increase investment cost, production cost and operating cost , It is difficult to ensure job safety, environment, hygiene requirements and other issues, to achieve the effect of simple process, low equipment requirements and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 1L four-neck round-bottomed flask equipped with a stirrer, thermometer, gas bubbler and vent, first replace the air with nitrogen. Add 500g (2.2mol) dicyclopentyldimethoxysilane into the flask, start stirring, cool the reaction flask to 20-25°C with an ice-water bath, and slowly pass in dried HCl gas through a bubbler, (actually pass The molar ratio is 2.2 times that of dicyclopentyldimethoxysilane). The aeration time is 10h and the aeration time is 1h, the aeration speed is 0.48mol / h, after the reaction is completed, stop the agitation, let it stand for stratification, remove the upper layer of methanol, and collect the distillate at 105-110°C under reduced pressure at 4-5mmHg absolute pressure. Obtained 452.5 g of dicyclopentyldichlorosilane (ESI-MS: m / z=237[M+H] + ), a purity of 98.2%, and a yield of 85.4%.
Embodiment 2
[0026] In a 1L four-neck round-bottomed flask equipped with a stirrer, thermometer, gas bubbler and vent, first replace the air with nitrogen. Add 500g (2.2mol) dicyclopentyldimethoxysilane into the flask, start stirring, cool the reaction flask to 20-25°C with an ice-water bath, and slowly pass in dried HCl gas through a bubbler, (actually pass The molar ratio is 2.2 times that of dicyclopentyldimethoxysilane). The aeration time is 10h and the aeration time is 1h, the aeration speed is 0.48mol / h, after the reaction is completed, stop the agitation, let it stand for stratification, remove the upper layer of methanol, and collect the distillate at 105-110°C under reduced pressure at 4-5mmHg absolute pressure. Obtained 434.6 g of dicyclopentyldichlorosilane (ESI-MS: m / z=237[M+H] + ), a purity of 98.0%, and a yield of 81.9%.
[0027] Taking the dicyclopentyl dichlorosilane done in Example 2 as a raw material to synthesize dicyclopentyl diethylaminosilane:
[0028] In a 2L four...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com