Nucleic acid multiplex PCR detection kit for 11 intestinal pathogenic bacteria and its application
A technology for detection kits and pathogenic bacteria, which is applied in the determination/inspection of microorganisms, resistance to vector-borne diseases, microorganisms, etc., can solve problems such as interactions, achieve high detection sensitivity, reduce detection costs, increase throughput and The effect of detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 111
[0049] Example 1 Nucleic acid multiplex PCR detection kit for 11 kinds of intestinal pathogenic bacteria
[0050] The kit consists of primer mix A, primer mix B, PCR reaction buffer, enzyme system, positive control, negative control and DEPC water.
[0051] PCR reaction solution A is a mixture of primer mixture A and PCR reaction buffer; PCR reaction solution B is a mixture of primer mixture B and PCR reaction buffer.
Embodiment 2
[0052] Operation and result determination of embodiment 2 kit
[0053] (1) Extraction of bacterial genomic DNA
[0054] Pick the feces the size of a grain of rice (take the mucus, pus and blood as much as possible), put them in a centrifuge tube with 0.5ml of normal saline, shake and mix well, and centrifuge at 13,000rpm for 2min, remove the supernatant, and precipitate for later use. Use the fecal nucleic acid extraction kit (product number: DP328) from Tiangen Biochemical Technology Co., Ltd. to extract nucleic acid in the sample processing area according to the instructions.
[0055] (2) Preparation of amplification system
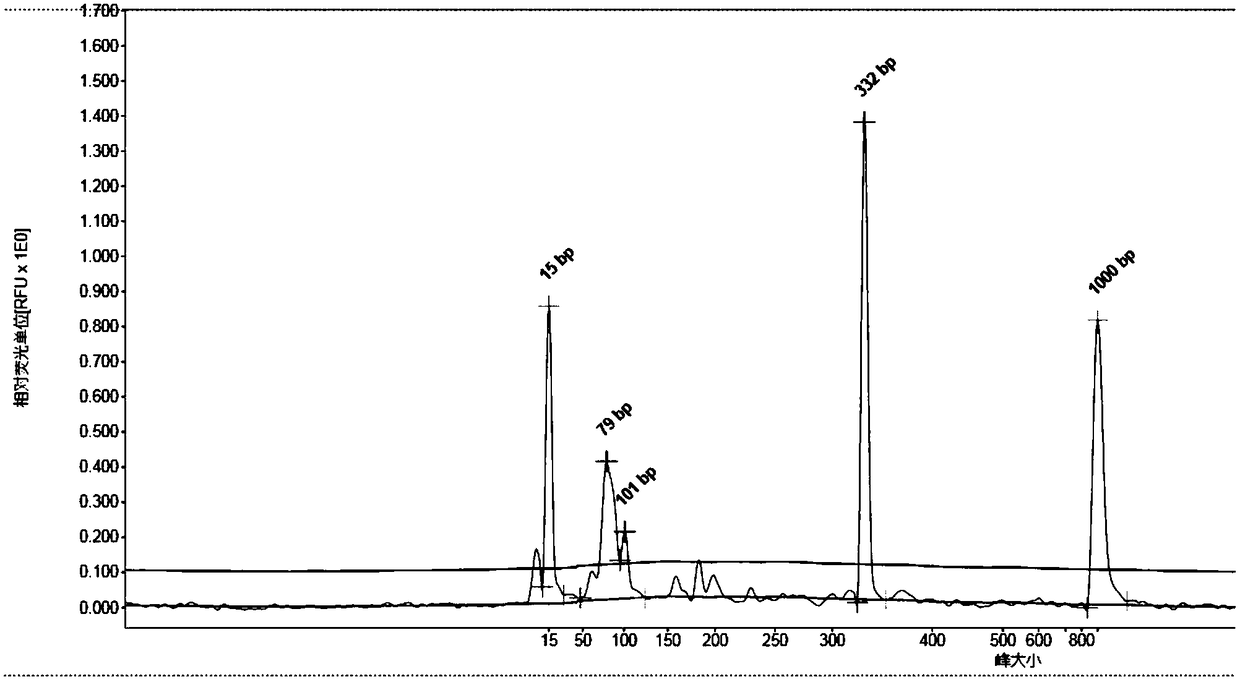
[0056] The following experiments were carried out using the kit of Example 1. After the components of the kit were completely dissolved at room temperature, they were quickly shaken and mixed. 25 μL PCR amplification system A reaction system is: 18 μL each of PCR reaction solution A, 2 μL enzyme system, 2-5 μL template (including nucleic acid extracte...
Embodiment 3
[0065] Embodiment 3 kit specificity experiment
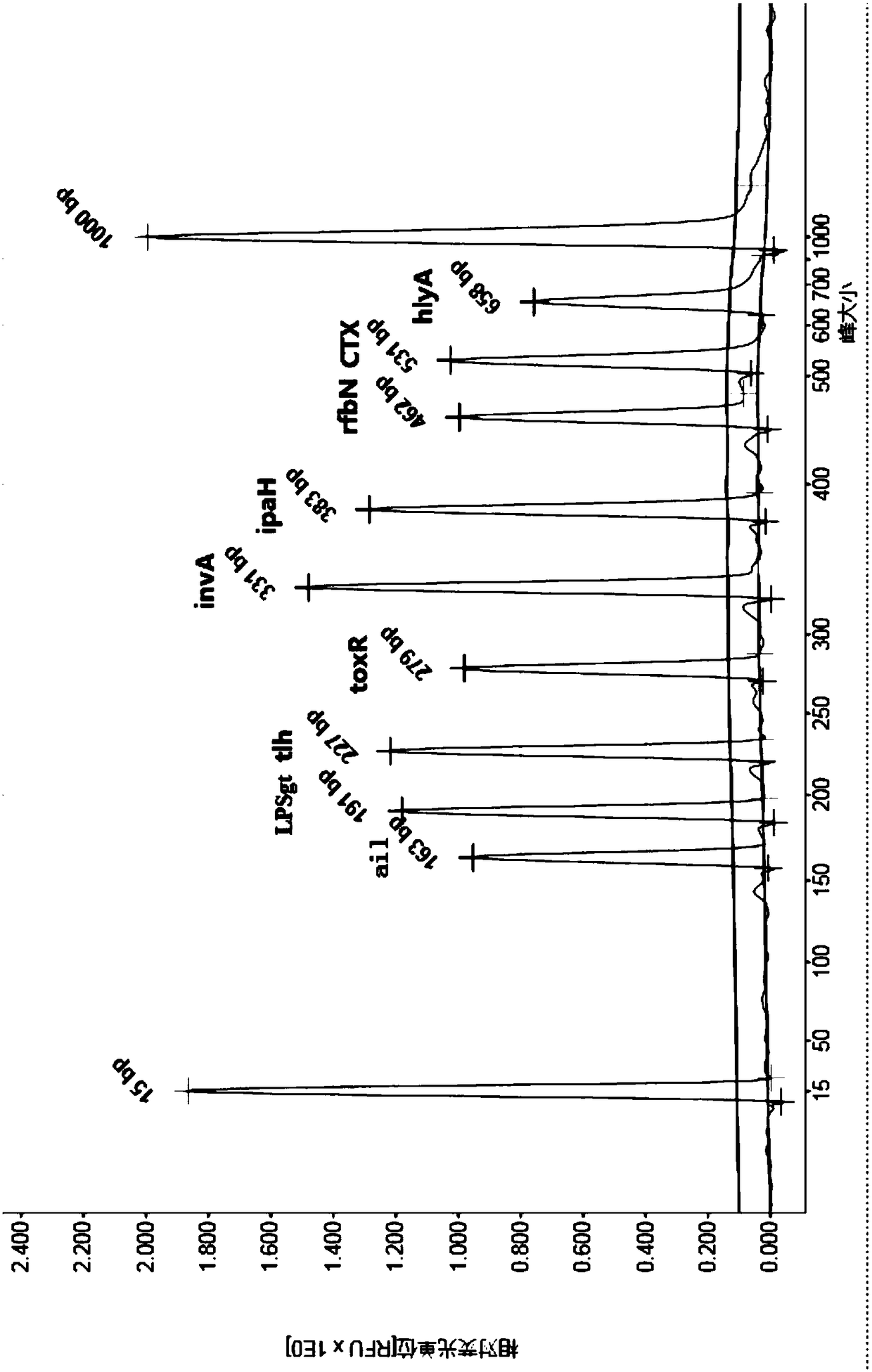
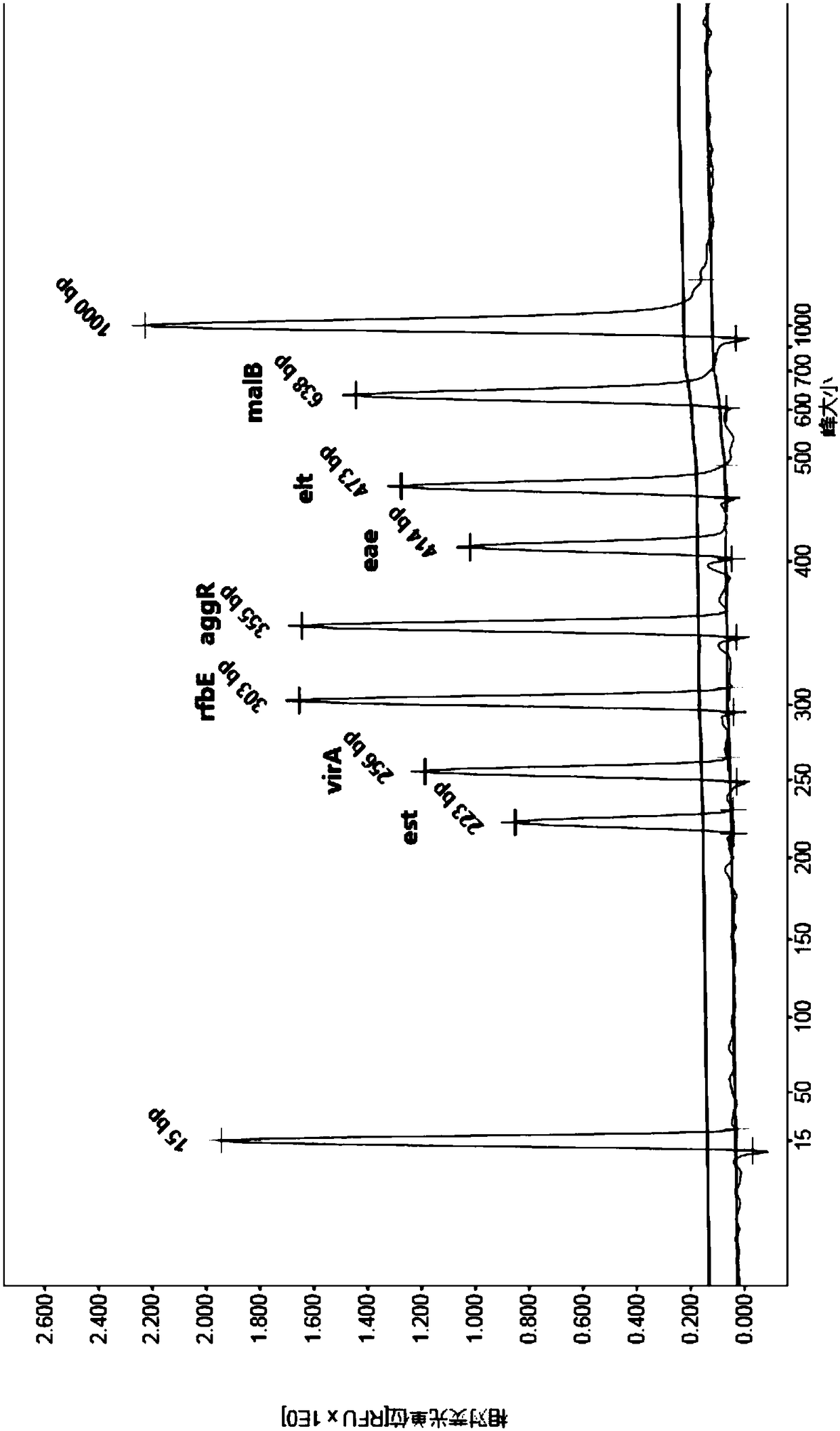
[0066] Select 8 enterovirus samples including coxsackievirus, poliovirus, echovirus, enteroadenovirus, norovirus, astrovirus, rotavirus group A, and zaravirus, as well as campylobacter jejuni and mononuclear virus 4 kinds of intestinal bacteria of cell proliferative Listeria, Vibrio alginolyticus, and Aeromonas, using the kit described in Example 1 to operate and determine the results according to the method described in Example 2, the detection results of the above 12 pathogens No characteristic peaks with peak sizes shown in Table 2 were shown, indicating that the kit of the present invention has good specificity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com