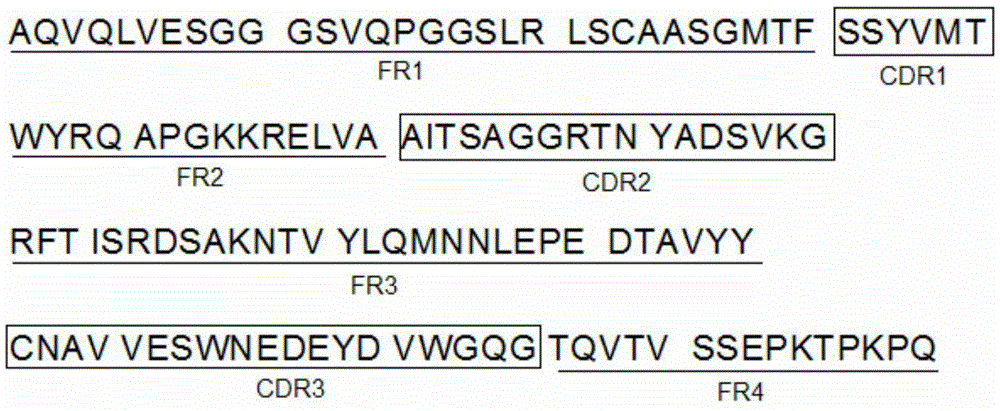
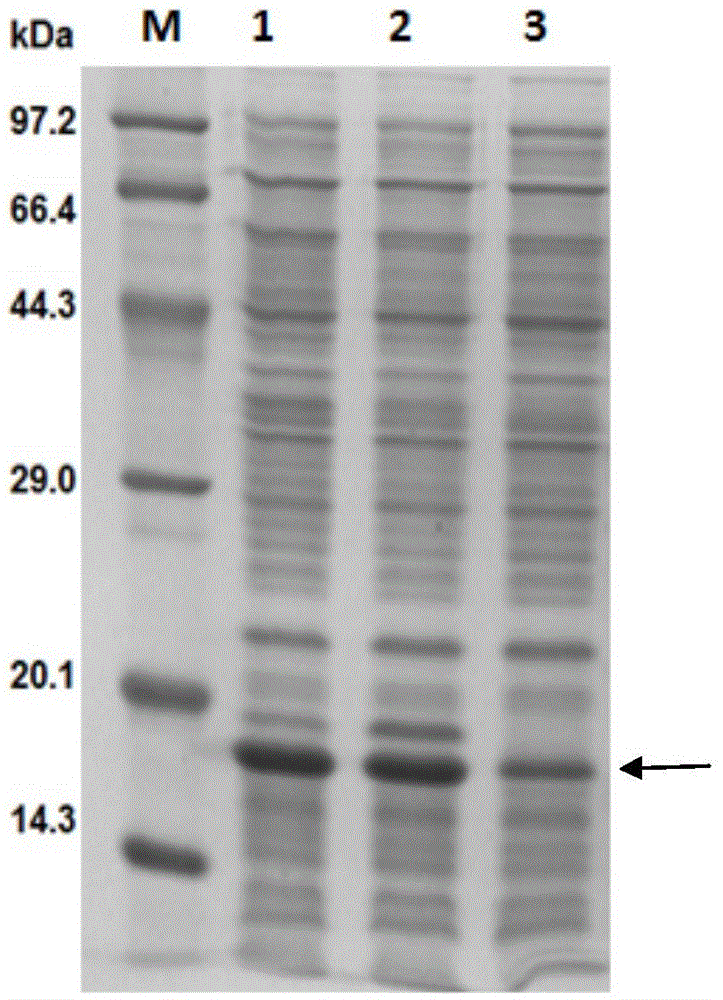
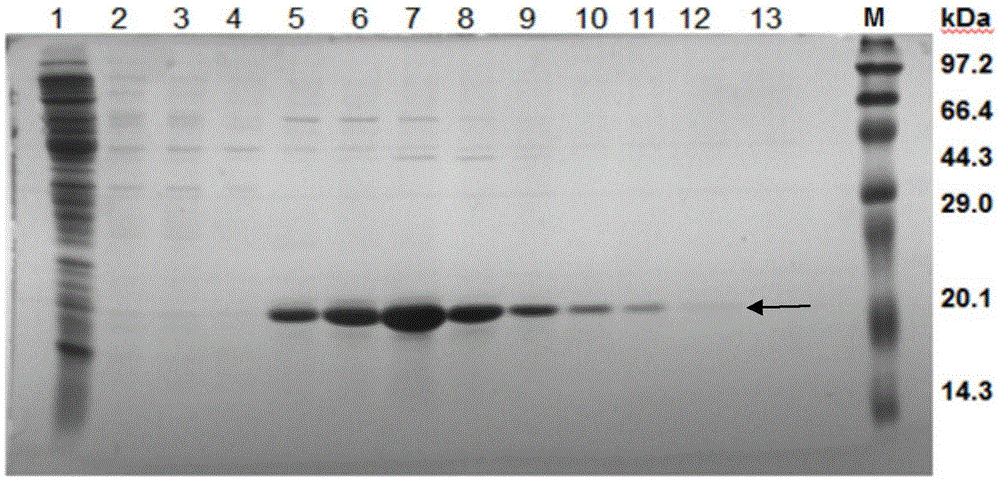
Anti-human amyloid beta-peptide nano antibody and application thereof
A nanobody and amyloid technology, applied in the fields of biochemistry and molecular biology, can solve the problems of increasing the cost of antibody preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Establishment of Nanobody Library
[0022] The phage display library used in the present invention is a non-immune library with T7 phage as the carrier, and the establishment steps are as follows:
[0023] (1) Total RNA was extracted from peripheral blood lymphocytes of 2-year-old male alpaca ( PuerLinkTMRNAMiniKit, LifeTechnologies: 12183018A); With UPprimer1 as a primer, the extracted total RNA was reverse-transcribed into cDNA, and two rounds of nested PCR were used to amplify the VHH gene; wherein the first round of PCR was based on the transcribed cDNA as a template, with UPprimer1 and DOWNprimer1 is the primer. After PCR amplification, a band with a size of 650-750bp is recovered, and then used as a template for the second round of PCR amplification. The upstream and downstream primers are UPprimer2 and DOWNprimer2 respectively, and a PCR product of 450-500bp is recovered. ;
[0024] UPprimer1:5'-GGTACGTGCTGTTGAACTGTTCC-3'
[0025] DOWNprimer1:5'-CTT...
Embodiment 2T7
[0030] The liquid amplification of embodiment 2T7 bacteriophage
[0031] (1) Activation of host bacteria: Inoculate Escherichia coli BLT5403 in 50ml of LB medium (containing 0.1mg / mL carbenicillin, referred to as Carb-LB), and culture overnight at 37°C and 170r / min to obtain the first-generation host bacteria;
[0032] (2) Inoculate 1% of the first-generation host bacteria in Carb-LB liquid medium, culture on a shaker, and reach OD 600 0.5-1.0, get the second generation host bacteria;
[0033] (3) Infect the second-generation host bacteria with T7 phage, culture at 200 r / min at 37°C for 2-3 hours, observe the bacteriolysis situation, and stop the cultivation immediately if the bacteriolysis is complete. The lysate was centrifuged at 8000 g for 10 min, and the supernatant was recovered, which was the amplified phage. Determine the titer.
Embodiment 3T7
[0034] The mensuration of embodiment 3T7 phage titer
[0035] (1) Pour the Carb-LB bottom medium into the culture dish, cool and solidify;
[0036] (2) Dissolve the top culture medium, cool it down, add Carb and keep it warm at 45°C;
[0037] (3) The amplified phages are diluted in gradient with sterile LB liquid medium;
[0038] (4) Mix 300 μL of the second-generation host bacteria with 50 μL of phage diluted in different gradients, add 4 mL of preheated Carb-top medium, quickly pour it into a plate with the bottom medium, and shake the plate to make it evenly distributed;
[0039] (5) Incubate upside down at 37°C for 3-4 hours until moderately sized plaques are seen;
[0040] (6) Select a plate with 50-500 plaques to count the plaques and calculate the phage titer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com