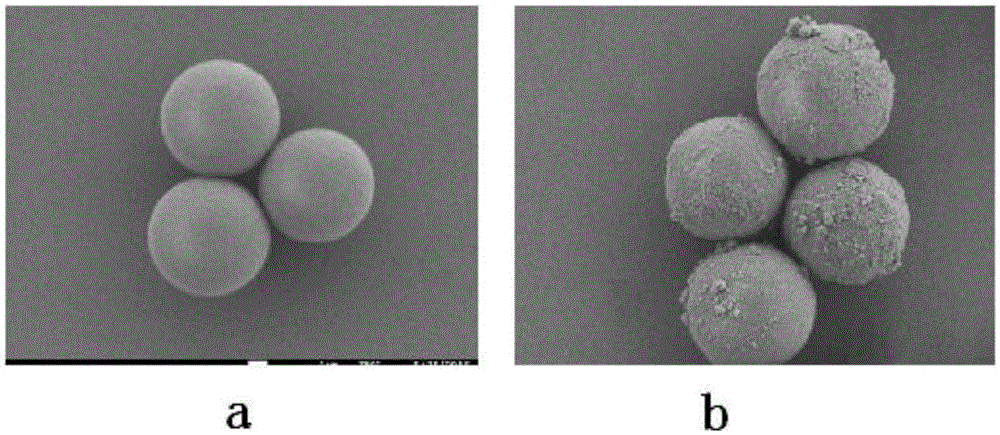
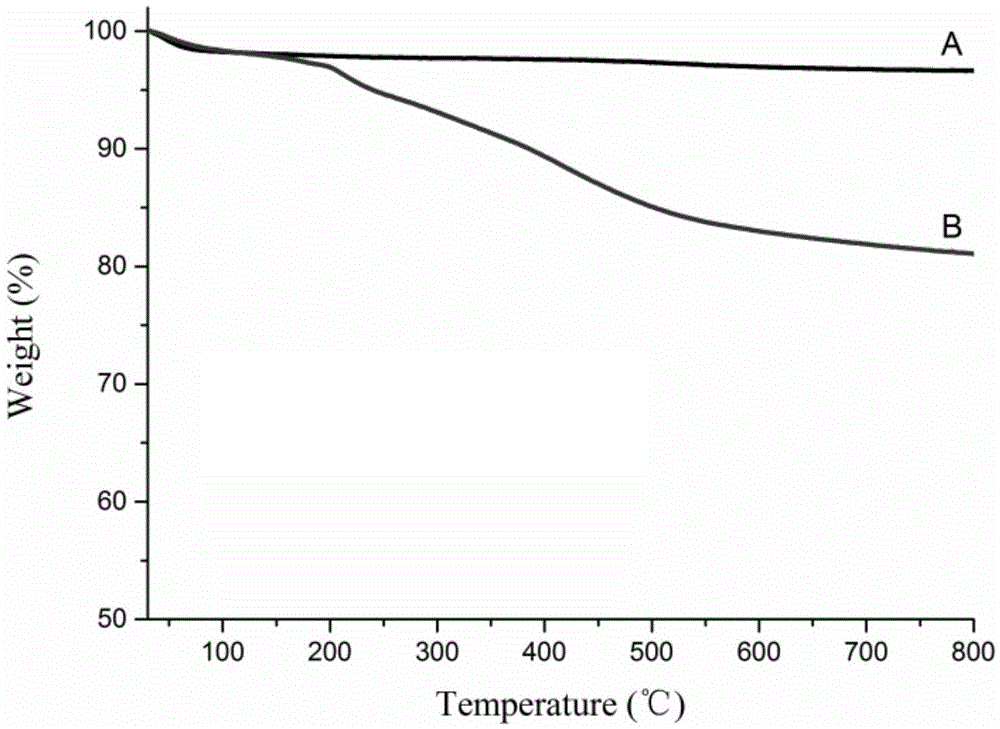
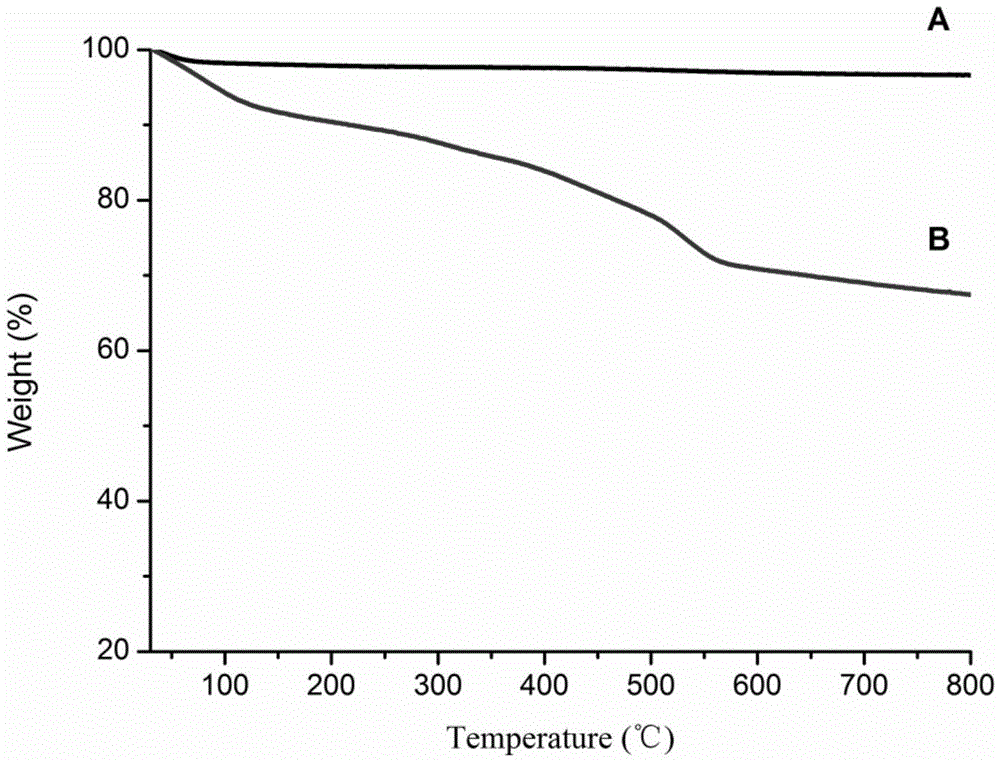
High-performance liquid chromatography filler with switchable chiral selectivity and preparing method thereof
A high-performance liquid chromatography, chiral technology, applied in the field of high-performance liquid chromatography packing and its preparation, to achieve the effects of good physical and chemical properties, high mechanical strength and lower detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0032] Example 1
[0033] Preparation of chiral stationary phase based on L-arabinose:
[0034] (1) Weigh 2g of silicon balls, react with 1mol / L HCl at 90°C for 3h, filter, wash with purified water to neutrality, and then dry at 150°C overnight for later use;
[0035] (2) Weigh 1.2 g of activated silicon balls, use toluene as a solvent, add 0.54 mL (3 mmol) of 3-aminopropyltrimethoxysilane (APTES), and react at room temperature for 16 hours. Centrifuge the toluene for 3 times and use toluene again. Suspend, add 0.24mL (3mmol) of pyridine, pipette 0.37mL (3mmol) of bromoisobutyryl bromide and dilute with 5mL of toluene, add dropwise to the reaction flask under nitrogen protection, react at 0°C for 2h, and react at room temperature overnight , Wash with toluene, water, methanol, dichloromethane, and acetone in sequence, and then vacuum dry at 110℃ for 3h;
[0036] (3) Using water, methanol and N,N-dimethylformamide in a volume ratio of 1:1:2 as a mixed solvent, the monomer N-isopropyla...
Example Embodiment
[0047] Example 2
[0048] Preparation of chiral stationary phase based on L-arabinose:
[0049] (1) Weigh 2g of silicon balls, react with 3mol / L HCl at 50-60℃ for 10h, filter, wash with purified water to neutrality, and then dry at 150℃ overnight for later use;
[0050] (2) Weigh 1.2 g of activated silicon balls, use toluene as a solvent, add 0.54 mL (3 mmol) of 3-aminopropyltriethoxysilane, and react at room temperature for 16 hours. Centrifuge the toluene three times and suspend again with toluene. Add 0.24mL (3mmol) of pyridine, pipette 0.37mL (3mmol) of bromoisobutyryl bromide and dilute with 5mL of toluene, add dropwise to the reaction flask under nitrogen protection, react at 0℃ for 2h, and react at room temperature overnight. Wash with toluene, water, methanol, dichloromethane and acetone in sequence, and then vacuum dry for 3h at 110℃;
[0051] (3) Mix water, methanol and N,N-dimethylformamide at a volume ratio of 1:1:2 as a mixed solvent, and add the monomer pure phenylboron...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com