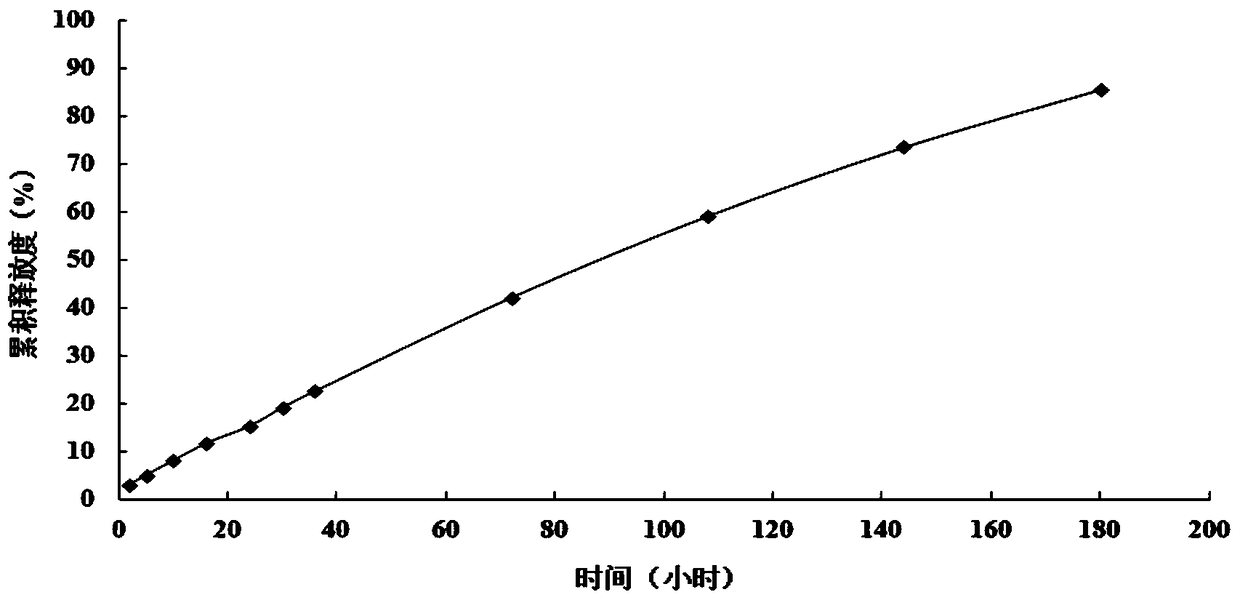
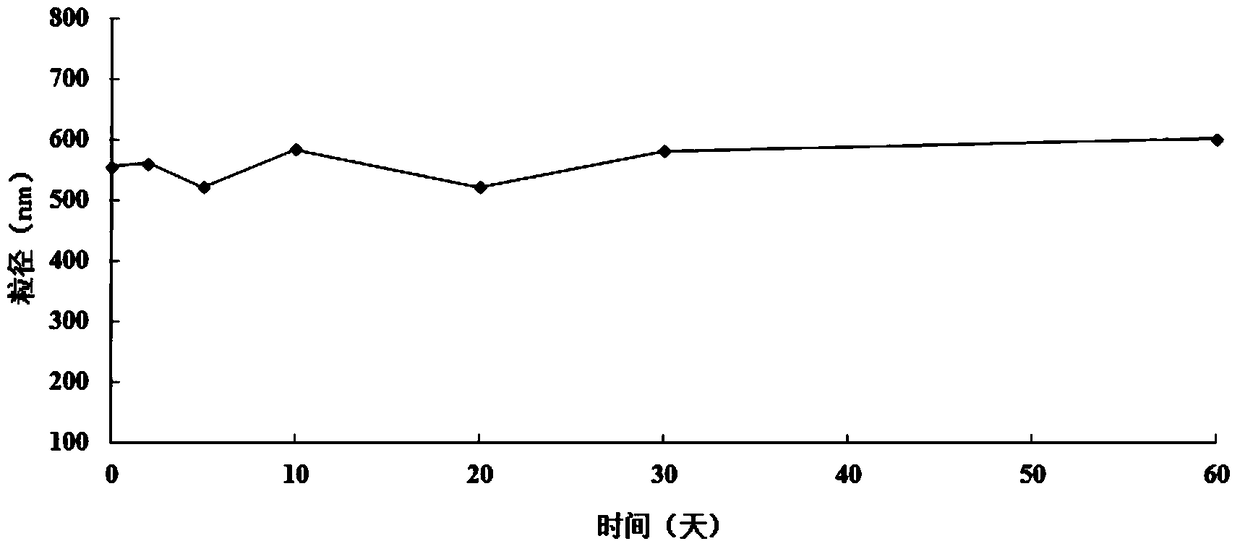
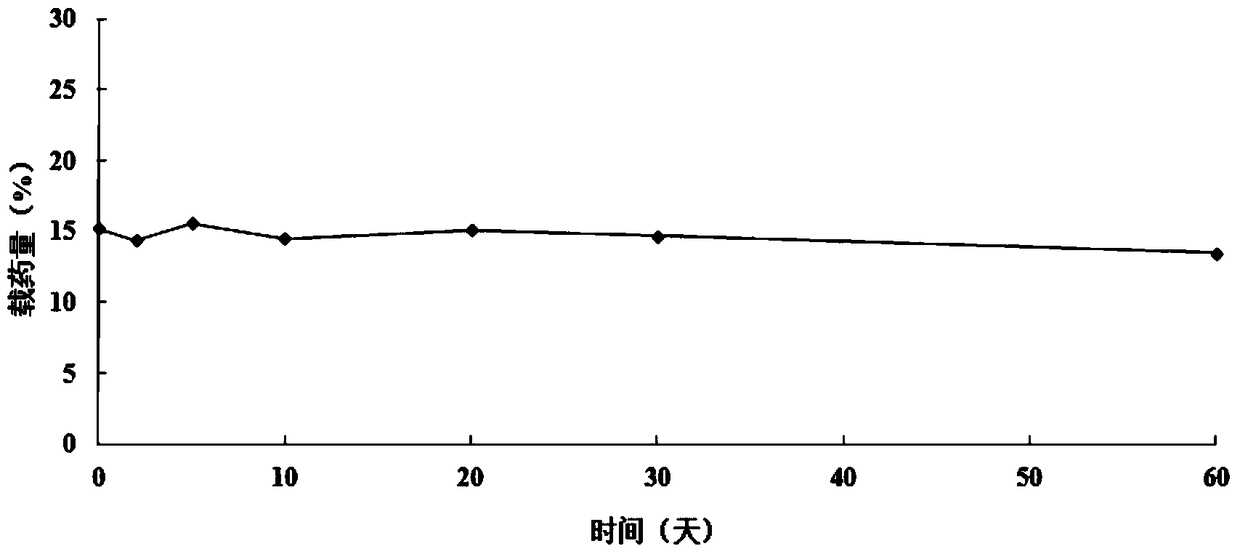
A supramolecular hydrogel system loaded with 10-hydroxycamptothecin and its preparation method
A technology of supramolecular hydrogel and hydroxycamptothecin, which is applied in the field of biomedical materials to achieve the effects of constant drug release rate, high drug loading and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of fatty acid esterified grafted polyvinyl alcohol:
[0032] Dissolve polyvinyl alcohol (Mw=10000, 2.2g) in 30ml of N-methylpyrrolidone, heat slowly to 30°C, then add caproyl chloride (0.001mol, 0.13g), lauroyl chloride (0.001mol, 0.22g) 1. Oleoyl chloride (0.001mol, 0.3g). After reacting at room temperature for 24 hours, a sufficient amount of water was added to precipitate out. After standing, filtering, washing twice and vacuum drying, 1.2 grams of fatty acid esterified grafted polyvinyl alcohol was obtained;
[0033] (2) Preparation of supramolecular hydrogel system loaded with 10-hydroxycamptothecin:
[0034] 50mg 10-hydroxycamptothecin and 0.17g step (1) gained fatty acid esterified grafted polyvinyl alcohol were dissolved in 20ml of dichloromethane and N-methylpyrrolidone mixed solvent with a volume ratio of 3:1 to obtain organic Solution A: Dissolve 0.85g of α-cyclodextrin in 20ml of water to obtain an aqueous solution of α-cyclodextrin; slowly ad...
Embodiment 2
[0036] (1) Synthesis of fatty acid esterified grafted polyvinyl alcohol:
[0037] Dissolve polyvinyl alcohol (Mw=50000, 2.2g) in 30ml of N-methylpyrrolidone, heat slowly to 50°C, then add lauroyl chloride (0.001mol, 0.22g), stearyl chloride (0.001mol, 0.3g ), after reacting at room temperature for 12 hours, a sufficient amount of water was added to precipitate out, and 1.1 g of fatty acid esterified grafted polyvinyl alcohol was obtained by standing, filtering, washing twice and vacuum drying;
[0038] (2) Preparation of supramolecular hydrogel system loaded with 10-hydroxycamptothecin:
[0039] 50mg 10-hydroxycamptothecin and 0.25g step (1) gained fatty acid esterified grafted polyvinyl alcohol were dissolved in 20ml of chloroform and N-methylpyrrolidone mixed solvent with a volume ratio of 3:1 to obtain organic Solution A: Dissolve 0.5g of α-cyclodextrin in 60ml of water to obtain an aqueous solution of α-cyclodextrin; slowly add organic solution A to the aqueous solution o...
Embodiment 3
[0041] (1) Synthesis of fatty acid esterified grafted polyvinyl alcohol:
[0042] Dissolve polyvinyl alcohol (Mw=80000, 2.2g) in 30ml N-methylpyrrolidone, heat slowly to 60°C, then add hexanoyl chloride (0.0005mol, 0.065g), stearyl chloride (0.0005mol, 0.15g ), after reacting at room temperature for 36 hours, a sufficient amount of water was added to precipitate out, and 1.0 g of fatty acid esterified grafted polyvinyl alcohol was obtained by standing, filtering, washing twice and vacuum drying;
[0043] (2) Preparation of supramolecular hydrogel system loaded with 10-hydroxycamptothecin:
[0044] 50mg 10-hydroxycamptothecin and 0.5g step (1) gained fatty acid esterified grafted polyvinyl alcohol are dissolved in the dichloromethane and N-methylpyrrolidone mixed solvent that 20ml volume ratio is 3:1, obtain organic Solution A; Dissolve 0.5g of α-cyclodextrin in 100ml of water to obtain an aqueous solution of α-cyclodextrin; slowly add the organic solution A to the aqueous sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com