Composition of a non-nucleoside reverse transcriptase inhibitor
A composition and polymer technology, which can be applied in the general field and can solve problems such as differences in the crystallization tendency of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
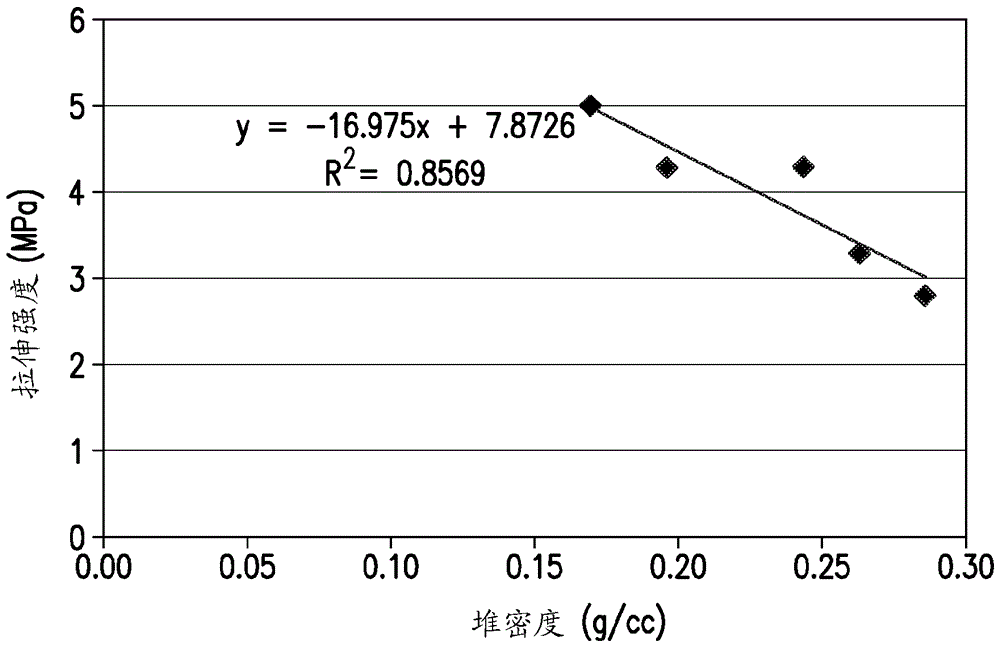
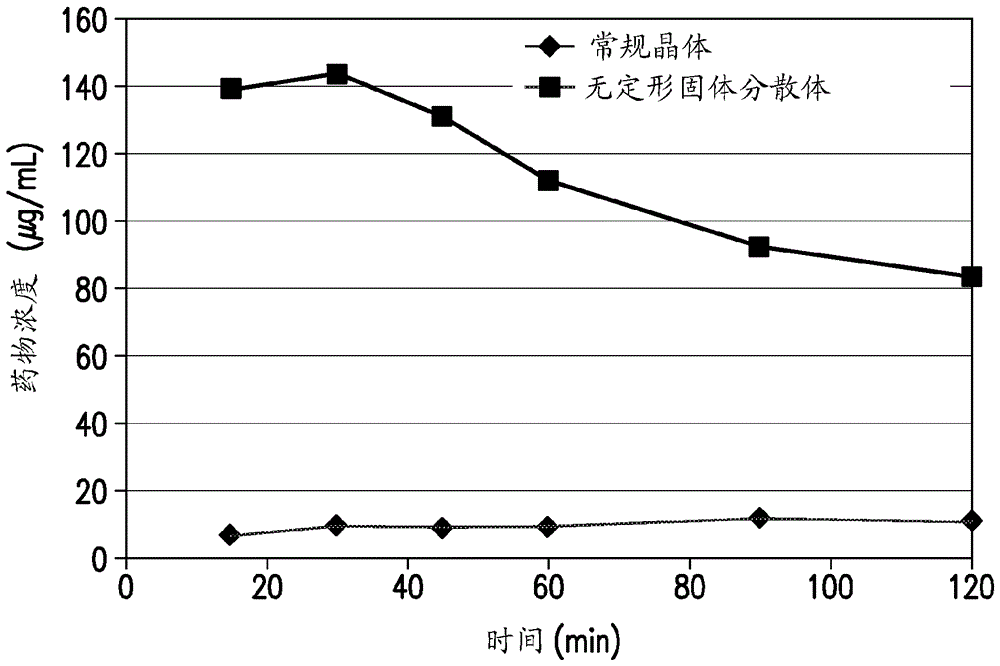
[0081] An example of the preparation of a spray-dried pharmaceutical formulation of Compound A is provided below. One goal in developing solid dispersion formulations was to achieve superior biological properties compared to conventional formulations containing crystalline Compound A. The biopharmaceutical comparison of spray-dried solid dispersion formulations containing increased concentrations of polymer was compared to conventional formulations containing the same amount of API. API is Compound A or a pharmaceutically acceptable salt thereof. Bioavailability was determined in vivo by administering to beagle dogs a test formulation of the active pharmaceutical ingredient (API) and / or other formulations at a dose of 1 mg / kg of API and then measuring the amount of API in serum or blood over time .
[0082] Preparation of spray-dried formulations
[0083] The spray-dried formulation comprises compound A (5-30% w / w); optional surfactants (1-10% w / w) such as SDS (sodium dod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com