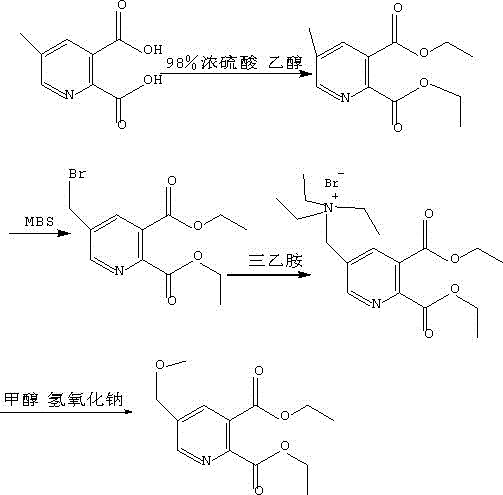
Synthesis method of 5-methoxy methyl pyridine-2,3-diethyl phthalate
A technology of diethyl diformate and methoxymethyl pyridine is applied in the field of organic chemical synthesis, can solve problems such as low yield, and achieve the effects of simple operation, mild reaction process and improved synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] First, add 300 mL of absolute ethanol to a 500 mL three-necked flask equipped with a thermometer and a reflux device, and then add 10 mL of 98% concentrated sulfuric acid at a rate of 2 mL / min at a stirring speed of 600 r / min. , after the temperature of the solution in the there-necked flask is naturally cooled to room temperature, then add 1.80g of 5-picoline-2,3-dicarboxylic acid in the there-necked flask, then heat the three-necked flask with an oil bath, so that the temperature of the solution in the there-necked flask is controlled at 110°C, keep warm for 1 hour, track the reaction by thin-layer chromatography, until the solution does not contain 5-picoline-2,3-dicarboxylic acid, the reaction ends, and 5-picoline-2,3-dicarboxylic acid dicarboxylate is obtained Ethyl ester solution; add 1.8g N-bromosuccinimide to the above solution, react for 1h, track the reaction by thin-layer chromatography, until the reaction solution no longer contains 5-picoline-2,3-dicarboxyli...
example 2
[0021] First, add 330 mL of absolute ethanol to a 500 mL three-necked flask equipped with a thermometer and a reflux device, and then add 13 mL of 98% concentrated sulfuric acid at a rate of 2 mL / min at a stirring speed of 700 r / min. , the temperature of the solution in the there-necked flask is naturally cooled to room temperature, then add 1.90g of 5-picoline-2,3-dicarboxylic acid in the there-necked flask, then heat the three-necked flask with an oil bath, so that the temperature of the solution in the there-necked flask is controlled at 120°C, keep warm for 1.5h, track the reaction by thin layer chromatography until the solution does not contain 5-methylpyridine-2,3-dicarboxylic acid, the reaction is over, and 5-methylpyridine-2,3-dicarboxylic acid is obtained Diethyl ester solution; add 1.9g N-bromosuccinimide to the above solution, react for 1.5h, track the reaction by thin-layer chromatography until the reaction solution no longer contains 5-picoline-2,3-di Diethyl form...
example 3
[0024] First, add 350 mL of absolute ethanol to a 500 mL three-necked flask equipped with a thermometer and a reflux device, and then add 15 mL of 98% concentrated sulfuric acid at a rate of 2 mL / min at a stirring speed of 800 r / min. , after the temperature of the solution in the there-necked flask is naturally cooled to room temperature, then add 2.00g of 5-methylpyridine-2,3-dicarboxylic acid in the there-necked flask, then heat the three-necked flask with an oil bath, so that the temperature of the solution in the there-necked flask is controlled at 130°C, keep warm for 2 hours, track the reaction by thin-layer chromatography until the solution does not contain 5-methylpyridine-2,3-dicarboxylic acid, and the reaction is completed to obtain 5-methylpyridine-2,3-dicarboxylic acid Ethyl ester solution; add 2.0g N-bromosuccinimide to the above solution, react for 2h, track the reaction by thin-layer chromatography until the reaction solution no longer contains 5-picoline-2,3-dic...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap