Pharmaceutical application of all-trans-retinoic acid
A technology of all-trans retinoic acid and its use, which is applied in the field of pharmaceutical application of all-trans retinoic acid, and can solve problems such as unknown mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
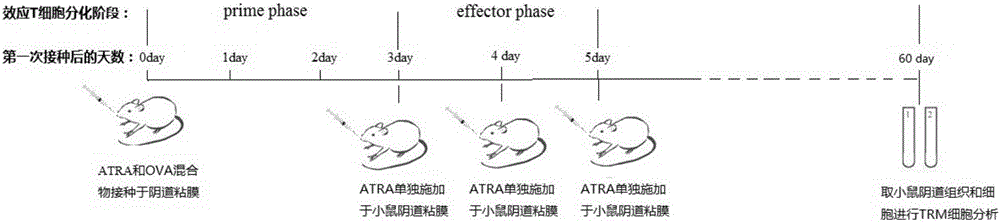
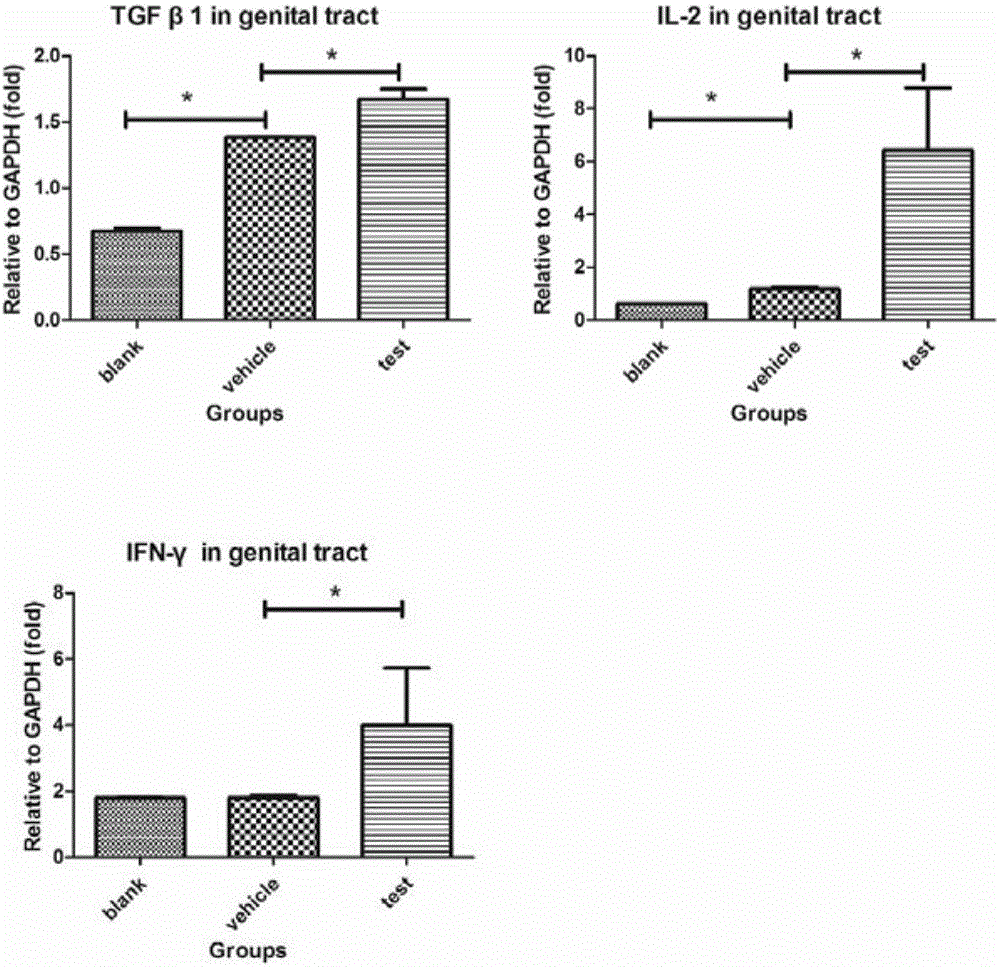
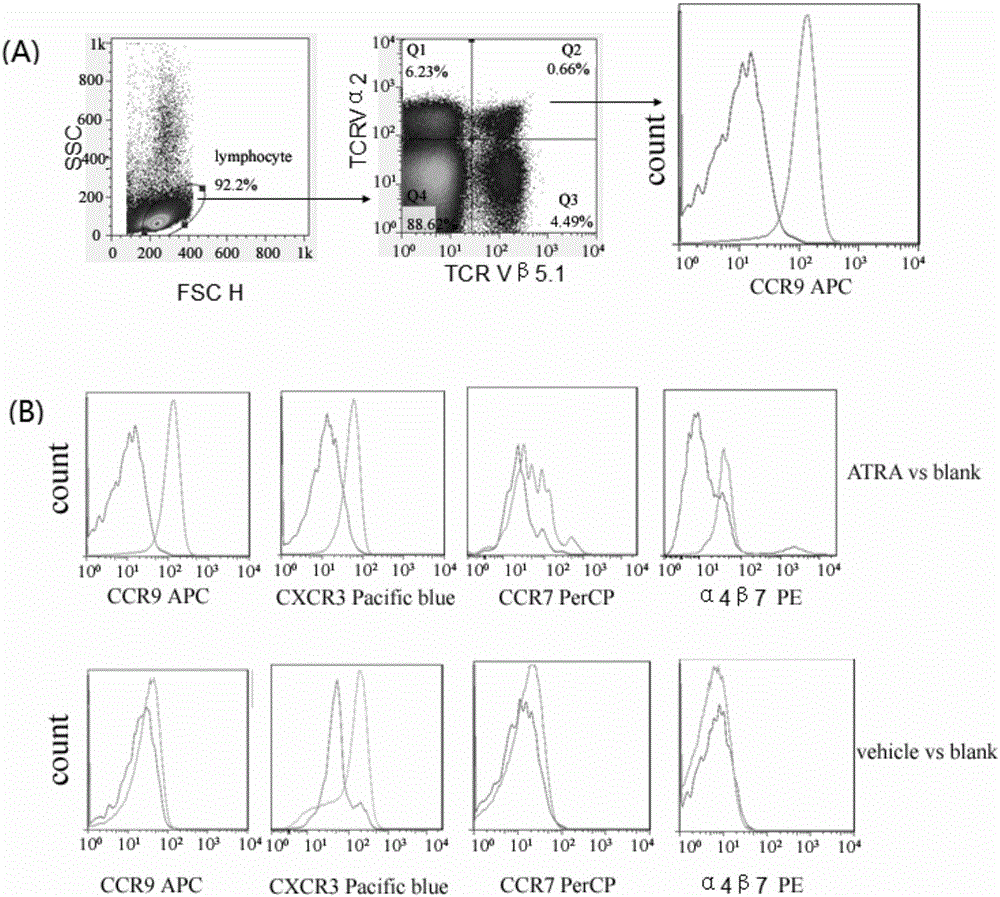
[0037] In this example, the inventors of the present invention mixed ATRA (all-trans retinoic acid) and chicken ovalbumin (OVA) and applied it to the vaginal mucosa of mice to observe the direction of OVA-specific CD8+ T cells towards reproduction. mucosal migration and eventual formation of T RM the process of.
[0038] Reagent
[0039] 1. ATRA, OVA and DMSO were purchased from SigmaAldrich;
[0040] 2. Experimental mice C57BL / 6 were purchased from Shanghai Slack Company, and OT-I mice were bred and bred in our laboratory.
[0041] method
[0042] 1. ATRA dissolution method: ATRA is dissolved in DMSO at a concentration of 30mg / ml, and stored at -80°C in the dark; when used, it is diluted 10 times with soybean oil to 3mg / ml, and applied to mice alone or mixed with 5mg / ml OVA mucous membrane site;
[0043] 2. Mice vaginal mucosa administration method: Use a pipette to absorb 30 μl of soybean oil to dissolve and dilute the ATRA or ATRA / OVA mixture, and gently push it into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com