A kind of preparation method of pyrazolyl acrylonitrile compound
A technology for pyrazolyl acrylonitrile and compounds, which is applied in the field of preparation of azole-based acrylonitrile compounds, can solve the problems of large amount of three wastes, low synthesis yield of intermediates, complicated post-treatment, etc. Good effect of "three wastes" emission and dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
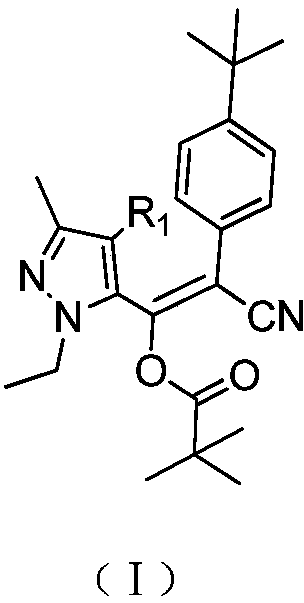
[0031] Z-2-(4-(tert-butyl)phenyl)-2-cyano-1-(1-ethyl-3-methyl-1hydro-5-pyrazolyl)vinyl pivalate (Table Synthesis of compound 1) in 1
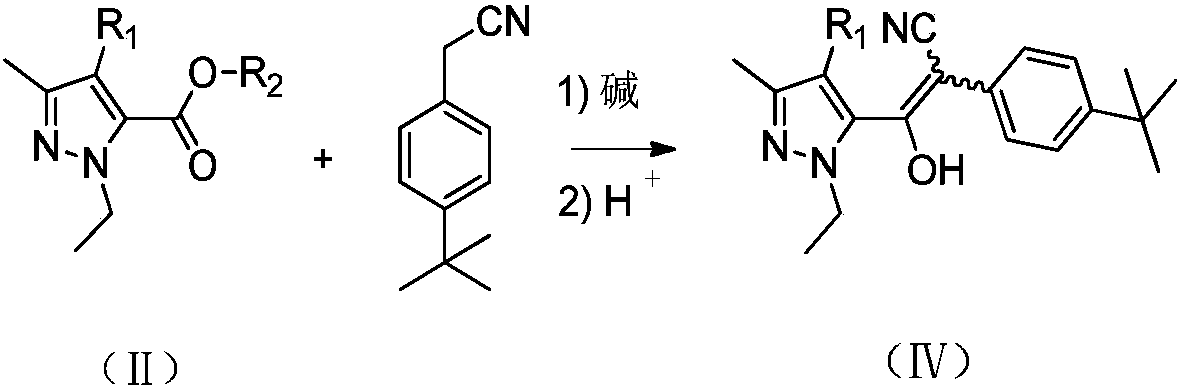
[0032] Add 43.7g (0.8mol) of sodium methoxide, 140g (0.8mol) of p-tert-butylphenylacetonitrile and 900g of toluene into a reaction flask equipped with a rectification tower, and heat up to 60°C. A solution of 170 g (1.0 mol) of methyl 1-ethyl-3-methyl-1-hydrogen-5-pyrazolecarboxylate and 300 g of toluene was slowly added dropwise, and the dropwise was completed in about 1 hour. After the dropwise addition, the temperature was raised to reflux. During the reaction, the low boilers with a boiling point lower than 65°C were continuously separated from the top of the tower and reacted for 5 hours to obtain 2-(4-(tert-butyl)phenyl)-1-(- 1-ethyl-3-methyl-1hydro-5-pyrazolyl)-2-cyanovinyl alcohol sodium salt suspension in toluene.
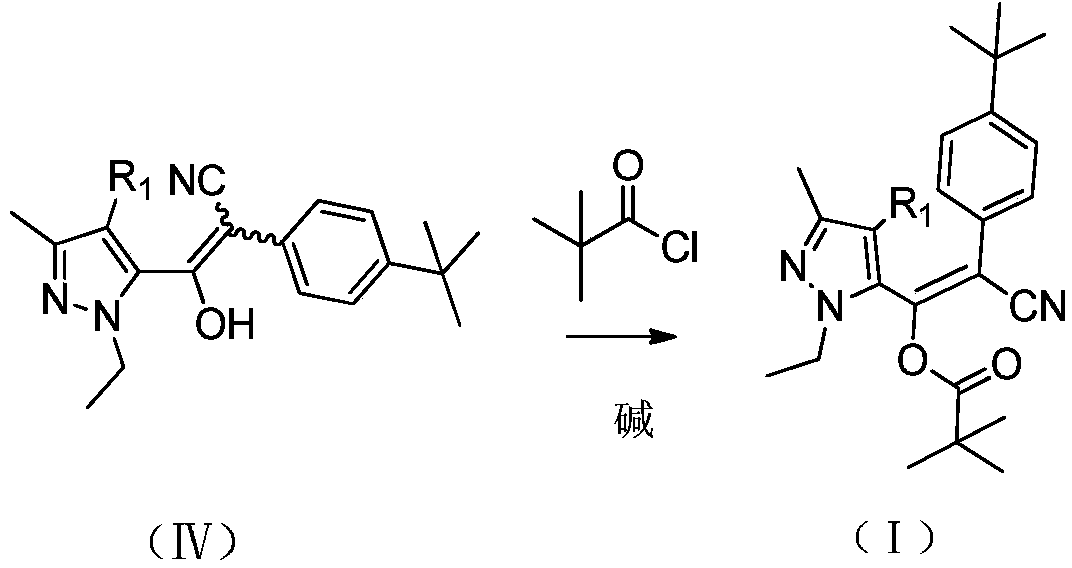
[0033] At 80-90° C., 98 g (0.8 mol) of pivaloyl chloride was added dropwise to the reaction mixture in the previous ste...
Embodiment 2
[0037] Z-2-(4-(tert-butyl)phenyl)-2-cyano-1-(1-ethyl-3-methyl-1hydro-5-pyrazolyl)vinyl pivalate (Table Synthesis of compound 1) in 1
[0038] In a reactor equipped with a rectification tower, 11Kg (20.2mol) of sodium methylate and 180Kg of toluene were added, and the temperature was raised to 100°C. Slowly add a solution of 33Kg (189mol) p-tert-butylphenylacetonitrile, 30Kg (177mol) 1-ethyl-3-methyl-1-hydrogen-5-pyrazolecarboxylic acid methyl ester and 50Kg toluene for about 1.5 hours. Finish. After the dropwise addition, the temperature was raised to reflux. During the reaction, the low boilers with a boiling point lower than 65°C were continuously separated from the top of the tower, and reacted for 6 hours to obtain 2-(4-(tert-butyl)phenyl)-1-(- 1-ethyl-3-methyl-1hydro-5-pyrazolyl)-2-cyanovinyl alcohol sodium salt suspension in toluene.
[0039] At 100-105° C., 24 Kg (197 mol) of pivaloyl chloride was added dropwise to the reaction mixture in the previous step, and the a...
Embodiment 3
[0041]Z-2-(4-(tert-butyl)phenyl)-2-cyano-1-(1-ethyl-3-methyl-4-chloro-1hydro-5-pyrazolyl)pivalic acid Synthesis of Vinyl Esters (Compound 5 in Table 1)
[0042] Add 54.6g (1.0mol) of sodium methoxide and 900g of toluene into the reaction flask equipped with a rectification tower, and raise the temperature to 80°C. Slowly add dropwise a solution of 205g (1.0mol) 4-chloro-1-ethyl-3-methyl-1hydrogen-5-pyrazolecarboxylic acid methyl ester, 175g (1.0mol) p-tert-butylphenylacetonitrile and 300ml of toluene , It takes about 2 hours to drip. After the dropwise addition, the temperature was raised to reflux. During the reaction, the low boilers with a boiling point lower than 65°C were continuously separated from the top of the tower, and reacted for 3 hours to obtain 2-(4-(tert-butyl)phenyl)-1-(4 - Chloro-1-ethyl-3-methyl-1hydro-5-pyrazolyl)-2-cyanovinyl alcohol sodium salt suspension in toluene.
[0043] At 105-110° C., 122 g (1.0 mol) of pivaloyl chloride was added dropwise to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com