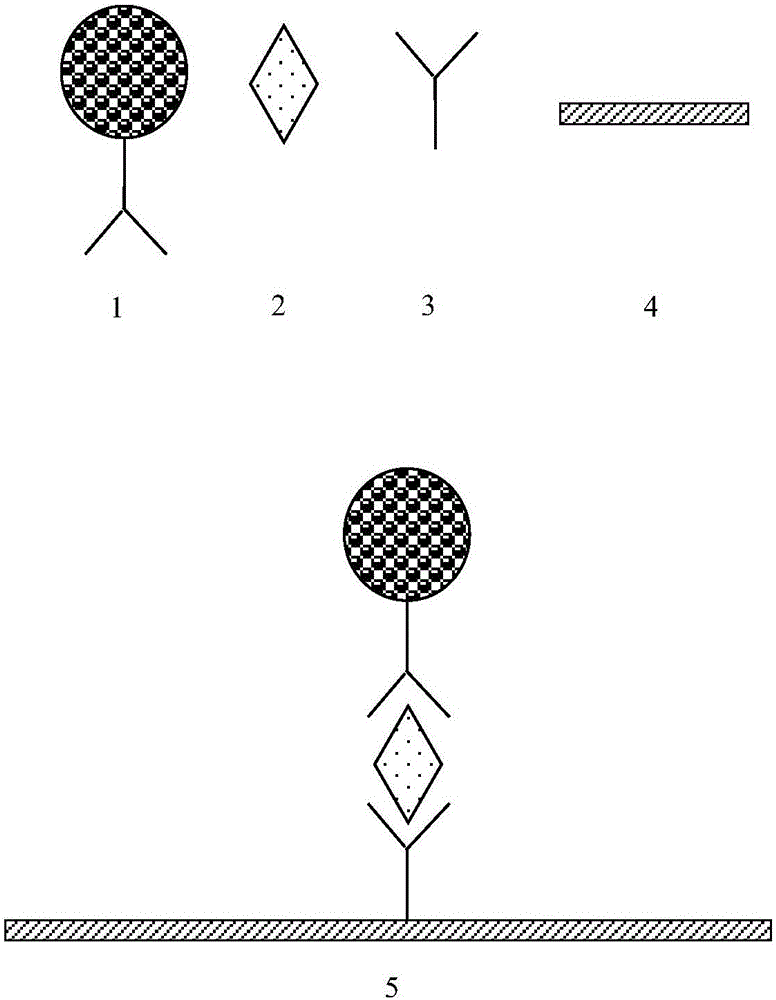Cup type time-resolved fluorescence D-dimer analysis method and reagent kit based on microspheres
A technology of time-resolved fluorescence and analysis method, which is applied in the field of cup type time-resolved fluorescence D-dimer analysis method and kit based on microspheres, which can solve the problem of unsatisfactory D-dimer detection precision and complicated detection steps , poor precision and other problems, to achieve the effect of reducing steric hindrance effect, shortening detection time and improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
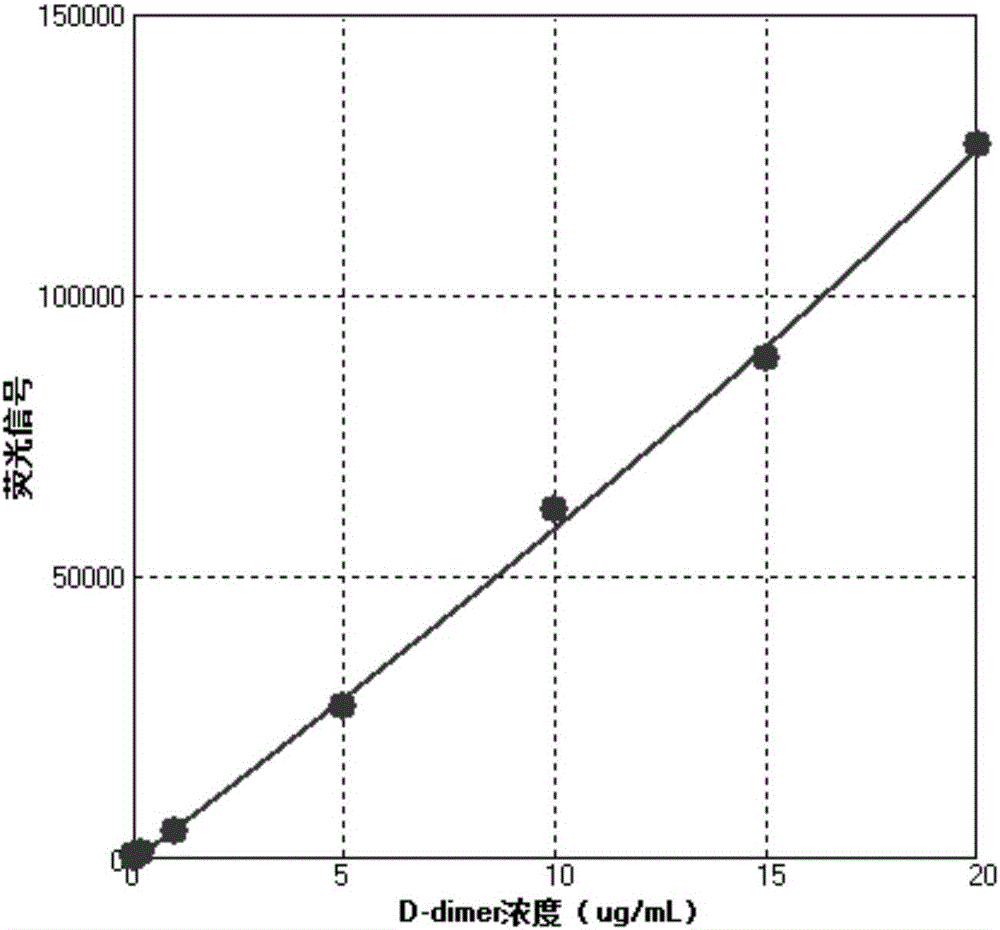
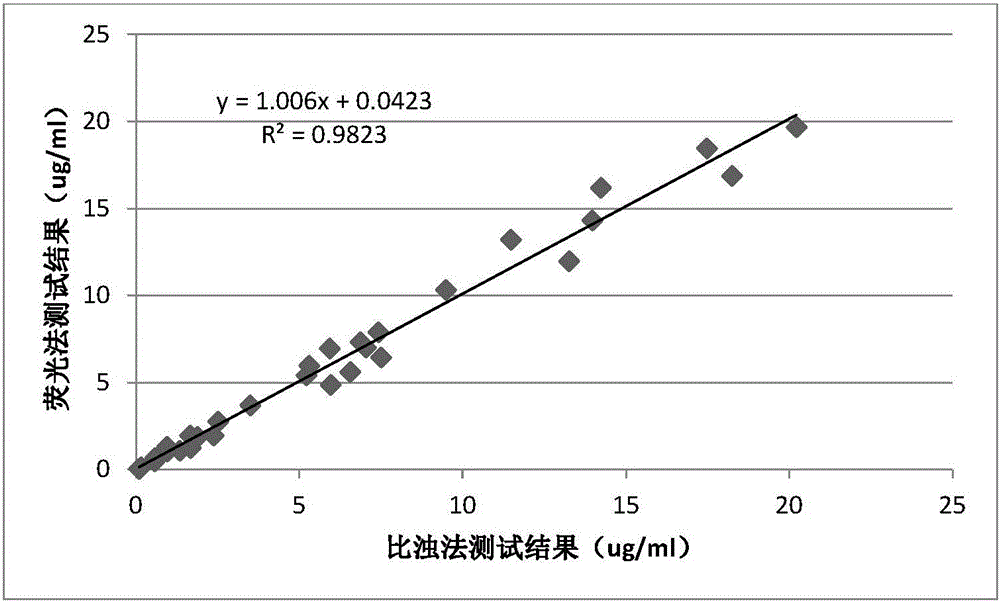
Image
Examples
Embodiment 1
[0055] 1. Preparation of detection cuvette
[0056] (1) Detection cuvette coating
[0057] D-dimer antibody 1401 (Medix Company) was diluted to 40 μg / ml with 0.2M phosphate buffer (pH 7.8), 100 μL / well, coated at 37° C. for 4 hours, and washed.
[0058] (2) Detect cuvette closure
[0059] Add 200 μL / well of blocking buffer to the reaction cup, block at 37°C for 4 hours, discard the blocking solution in the coated reaction cup, and pat dry.
[0060] (3) Detection of cuvette drying
[0061] The sealed cuvette was placed in a 37° C. drying oven with a humidity lower than 30% for 8 hours, sealed and dried for storage.
[0062] 2. Preparation of fluorescently labeled antibodies
[0063] (1) Pretreatment of fluorescent particles
[0064] Take 1mg of carboxyl time-resolved fluorescent microspheres (200nm, 0.1ml, 10mg / mL, Bangslab company), add 60ul 500mM MES (2-(N-morpholine)ethanesulfonic acid) buffer, pH 6.0, add 0.2mg 1 -(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochlor...
Embodiment 2
[0091] (1) Detection cuvette coating
[0092] D-dimer antibody 1401 (Medix Company) was diluted to 40 μg / ml with 0.2M phosphate buffer (pH 7.8), 100 μL / well, coated at 37° C. for 4 hours, and washed.
[0093] (2) Detect cuvette closure
[0094] Add 200 μL / well of blocking buffer to the reaction cup, block at 37°C for 4 hours, discard the blocking solution in the coated reaction cup, and pat dry.
[0095] (3) Detection of cuvette drying
[0096] The sealed cuvette was placed in a 37° C. drying oven with a humidity lower than 30% for 8 hours, sealed and dried for storage.
[0097] 2. Preparation of fluorescently labeled antibodies
[0098] (1) Pretreatment of fluorescent particles
[0099] Take 1mg of carboxyl time-resolved fluorescent microspheres (200nm, 0.1ml, 10mg / mL, Bangslab company), add 60ul 500mM MES (2-(N-morpholine)ethanesulfonic acid) buffer, pH 6.0, add 0.2mg 1 -(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), add 0.4mg N-hydroxysuccinimide (NHS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com