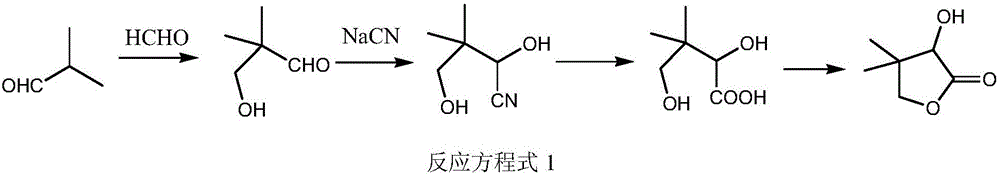
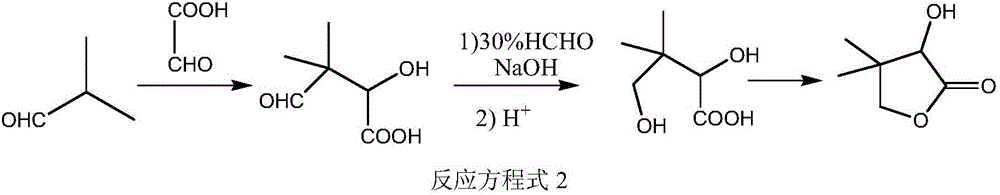
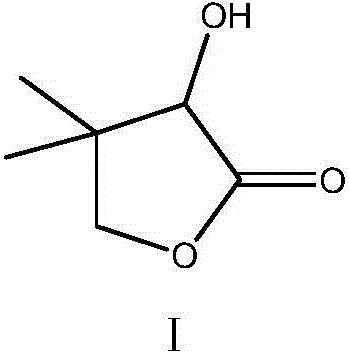
Environment-friendly synthesis method of D-calcium pantothenate intermediate D, L-pantoyl lactone
A pantolactone and lactonization technology, applied in organic chemistry and other directions, can solve problems such as large amount of formaldehyde and sodium hydroxide used, high COD in wastewater, unfavorable environmental protection, etc., and achieves high raw material atom economy and separation. Fewer processes and lower energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of D, L-pantolactone
[0045] Add 19.0 grams (0.1 moles) of 40 wt% glyoxylic acid and 0.5 grams of triethylamine to a 250 milliliter four-necked flask, and add 7.0 grams (0.1 moles) of isobutyraldehyde dropwise between 20 and 25 ° C. React at 30°C for 3 hours. Transfer the resulting reaction liquid to a 250 ml stainless steel pressure vessel, add 30 g of 1,2-dichloroethane, 0.2 g of 5% palladium carbon catalyst, pass in hydrogen, keep at 2-3 atmospheric pressure, stir and catalyze at 20-25°C The hydrogenation reaction was carried out for 4 hours. Filter and recover the catalyst. Add 0.3 g of 75% sulfuric acid (accounting for 5.71% of isobutyraldehyde after conversion) to the filtrate, and react at 80-85°C for 3 hours; Dichloroethane extraction, 20 grams each time, combined organic phases, washed with 10 grams of 1.0% sodium carbonate aqueous solution, washed with 10 grams of saturated sodium chloride aqueous solution, recovered 1,2-dichl...
Embodiment 2
[0046] Embodiment 2: the preparation of D, L-pantolactone
[0047] Add 19.6 grams (0.11 moles) of 40% glyoxylic acid and 0.4 grams of piperidine to a 250 ml four-neck flask, add 7.0 grams (0.1 moles) of isobutyraldehyde dropwise between 15 and 20 °C, after the drop is completed, set the temperature at 20 to 25 °C React for 3 hours. Transfer the resulting reaction liquid to a 250 ml stainless steel pressure vessel, add 40 g of n-butyl acetate, 0.2 g of 5% palladium carbon catalyst, feed hydrogen, keep at 2-3 atmospheric pressure, and stir at 20-25 ° C for catalytic hydrogenation reaction 4 Hour. Filter and recover the catalyst. Add 0.3 g of 85% sulfuric acid (accounting for 5.04% of isobutyraldehyde after conversion) to the filtrate, and react at 90-95°C for 3 hours; after the reaction is completed, cool to 15-20°C, separate layers, and use n-butyl acetate for the water layer Extraction, 20 grams each time, combined organic phases, washed with 10 grams of 1.0% aqueous sodium...
Embodiment 3
[0048]Embodiment 3: D, the preparation of L-pantolactone
[0049] Add 19.6 grams (0.11 moles) of 40% glyoxylic acid and 0.4 grams of tri-n-butylamine to a 250 milliliter four-necked flask, and add 7.0 grams (0.1 moles) of isobutyraldehyde dropwise between 20 and 25°C. React at 30°C for 3 hours. Transfer the resulting reaction liquid to a 250ml stainless steel pressure vessel, add 40g of 1,2-dichloroethane, 0.5g of 50% Raney nickel catalyst, feed hydrogen, keep stirring at 30-35°C under 3-4 atmosphere pressure The catalytic hydrogenation reaction was carried out for 4 hours. Filter and recover the catalyst. Add 0.3 g of 75% sulfuric acid to the filtrate, react at 80-85°C for 3 hours; after the reaction is complete, cool to 15-20°C, separate layers, extract the water layer with 1,2-dichloroethane, 20 g each time, combine The organic phase was washed with 10 g of 1.0% sodium carbonate aqueous solution and 10 g of saturated sodium chloride aqueous solution. After the organic ph...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap