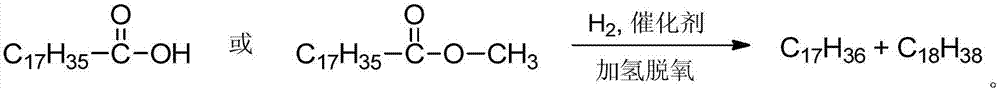A method for preparing long-chain alkane fuels by catalyzing the hydrodeoxygenation of fatty acids or fatty acid esters
A fatty acid ester and hydrodeoxygenation technology, which is used in the preparation of liquid hydrocarbon mixtures, biological raw materials, petroleum industry, etc., can solve the problems of difficult separation of reaction products and systems, chain scission loss of alkane products, and low catalytic efficiency, etc. To achieve the effect of easy separation, reduced energy consumption, and easy implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of long-chain alkane fuel by ruthenium-catalyzed hydrogenation-deoxygenation of methyl stearate.
[0022] In 80mL stainless steel autoclave, add methyl stearate (200mg, 0.67mmol), catalyst Ru / HZSM-5 (150mg, Ru 1.0wt.%, Si / Al=25), H 2 O (10mL) with H 2 H 2 After the pressure was raised to 3.0 MPa to ensure that the autoclave was airtight, it was placed in a heating mantle at 200° C. and stirred for 8 hours. During the reaction, the maximum pressure in the kettle can reach about 6MPa; after the reaction is completed, the pressure in the kettle will drop to about 3MPa after cooling to room temperature.
[0023] The reaction solution was extracted with cyclohexane, and the extracted organic phase was analyzed and quantified by gas-mass mass. The analysis results showed that the conversion rate of methyl stearate was 91%, the yield rate of heptadecane was 64%, the yield rate of octadecane was 13%, and the yield rate of stearic acid was 0%.
Embodiment 2
[0030] Example 2: Platinum-catalyzed hydrogenation-deoxygenation of methyl stearate to prepare long-chain alkane fuels.
[0031] In 80mL stainless steel autoclave, add methyl stearate (200mg, 0.67mmol), catalyst Pt / HZSM-5 (150mg, Pt 1.0wt.%, Si / Al=25), H 2 O (10mL) with H 2 H 2 After the pressure was raised to 3.0 MPa to ensure that the autoclave was airtight, it was placed in a heating mantle at 200° C. and stirred for 8 hours. During the reaction, the maximum pressure in the kettle can reach about 6MPa; after the reaction is completed, the pressure in the kettle will drop to about 3MPa after cooling to room temperature.
[0032] The reaction solution was extracted with cyclohexane, and the extracted organic phase was analyzed and quantified by gas-mass mass. The analysis results showed that the conversion rate of methyl stearate was 81%, the yield rate of heptadecane was 0%, the yield rate of octadecane was 1%, and the yield rate of stearic acid was 69%.
Embodiment 3
[0033] Example 3: Preparation of long-chain alkane fuel by ruthenium-catalyzed hydrogenation-deoxygenation of methyl stearate.
[0034] In 80mL stainless steel autoclave, add methyl stearate (200mg, 0.67mmol), catalyst Ru / HZSM-5 (150mg, Ru 1.0wt.%, Si / Al=25), H 2 O (10mL) with H 2 H 2 After the pressure was raised to 3.0 MPa to ensure that the autoclave was airtight, it was placed in a heating mantle at 180° C. and stirred for 8 hours. During the reaction, the pressure inside the kettle can reach up to about 5.5MPa; after the reaction is finished, cool to room temperature, and the pressure inside the kettle drops to about 3MPa.
[0035] The reaction solution was extracted with cyclohexane, and the extracted organic phase was analyzed and quantified by gas-mass mass. The analysis results showed that the conversion rate of methyl stearate was 24%, the yield rate of heptadecane was 17%, the yield rate of octadecane was 2%, and the yield rate of stearic acid was 0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com