Novel aspartic acid derivative, preparation method and use thereof in solid-phase polypeptide synthesis
A technology of aspartic acid and derivatives, which is applied in the preparation of carbamic acid derivatives, organic compounds, hydrogenated polysulfides/polysulfides, etc., which can solve the problems of expensive additives, low efficiency of Fmoc removal, and effect effects Larger issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
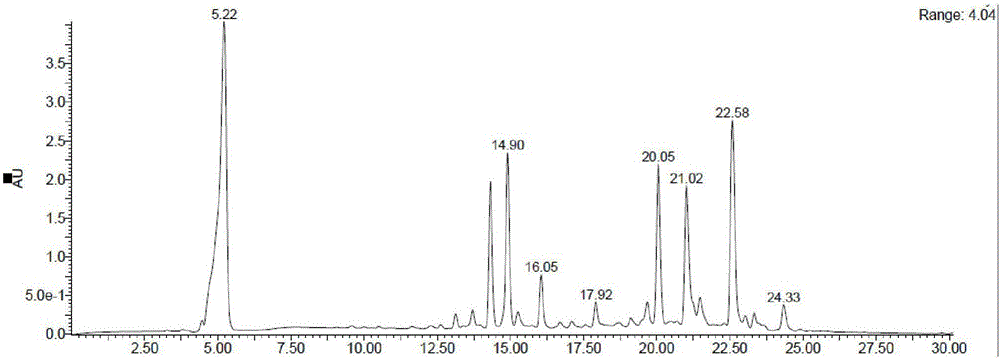
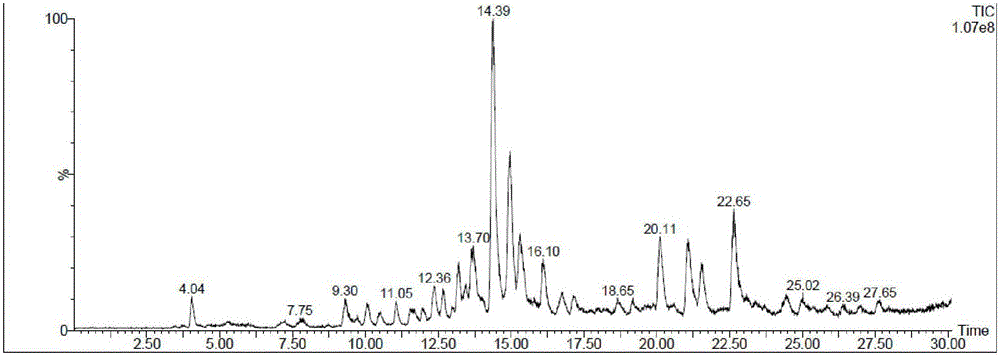
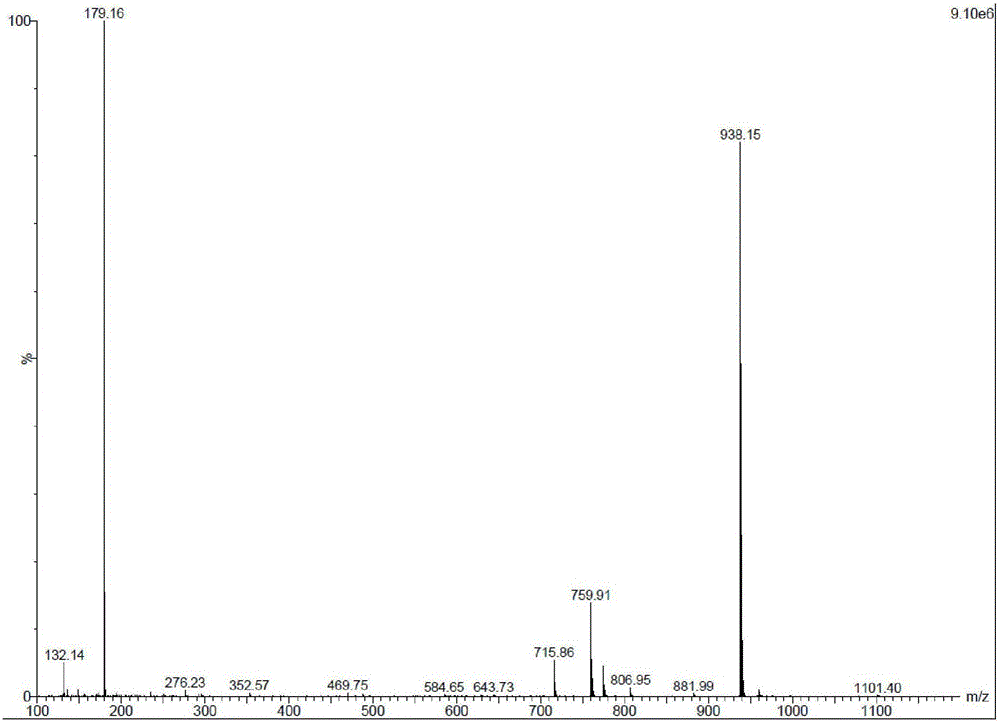
Image
Examples
Embodiment Construction
[0020]
[0021] (1) Synthesis of compound 5: Dissolve compound 1 (1g, 3mmol) in N,N-dimethylformamide (10ml), add N,N-diisopropylethylamine (0.8ml, 4.5mmol) and propenyl bromide (0.34ml, 4.44mmol). The reaction was stirred overnight at room temperature, and the reaction was monitored by thin-layer chromatography. After the reaction was completed, acid was added to terminate the reaction. Add ethyl acetate to dilute the reaction, wash the organic layer with water and saturated brine respectively, dry the organic phase with anhydrous magnesium sulfate, filter with suction, spin dry and pass through a silica gel column, the eluent is petroleum ether:ethyl acetate (9:1 ), finally obtained a colorless oil with a yield of 95%. 1 H NMR (400MHz, CDCl3) δ5.87 (dddd, J = 5.3, 5.6, 10.2, 16.9Hz, 1H), 5.48 (br d, J = 8.4Hz, 1H), 5.31 (dq, J = 16.8, 1.5Hz ,1H),5.22(dq,J=10.5,1.1Hz,1H),4.65(ddt,J=13.0,5.5,1.5Hz,1H),4.63(ddt,J=13.4,
[0022] 5.7,1.5Hz,1H),4.54(dt,J=4.5,8.8Hz,1H),2.92(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com