Fucoidan-based theragnostic Composition
A technology for fucoidan and conjugates, which is applied in the field of preparation of diagnostic and therapeutic agent compositions, can solve the problems of inability to obtain photodynamic therapy effects, increased steps and costs, and difficulty in mass production of conjugates, and achieves inhibition of smooth muscle cells. proliferative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
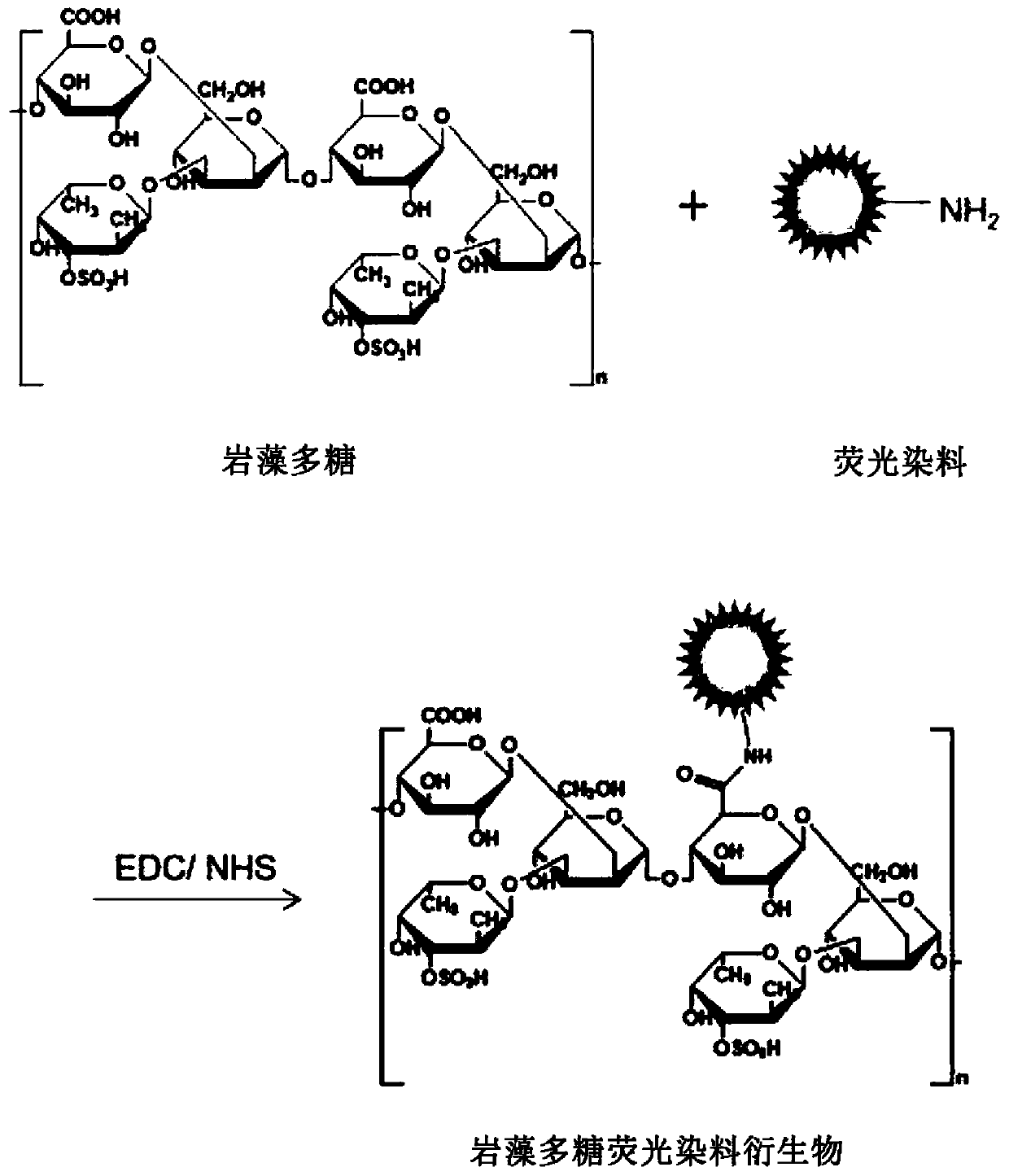
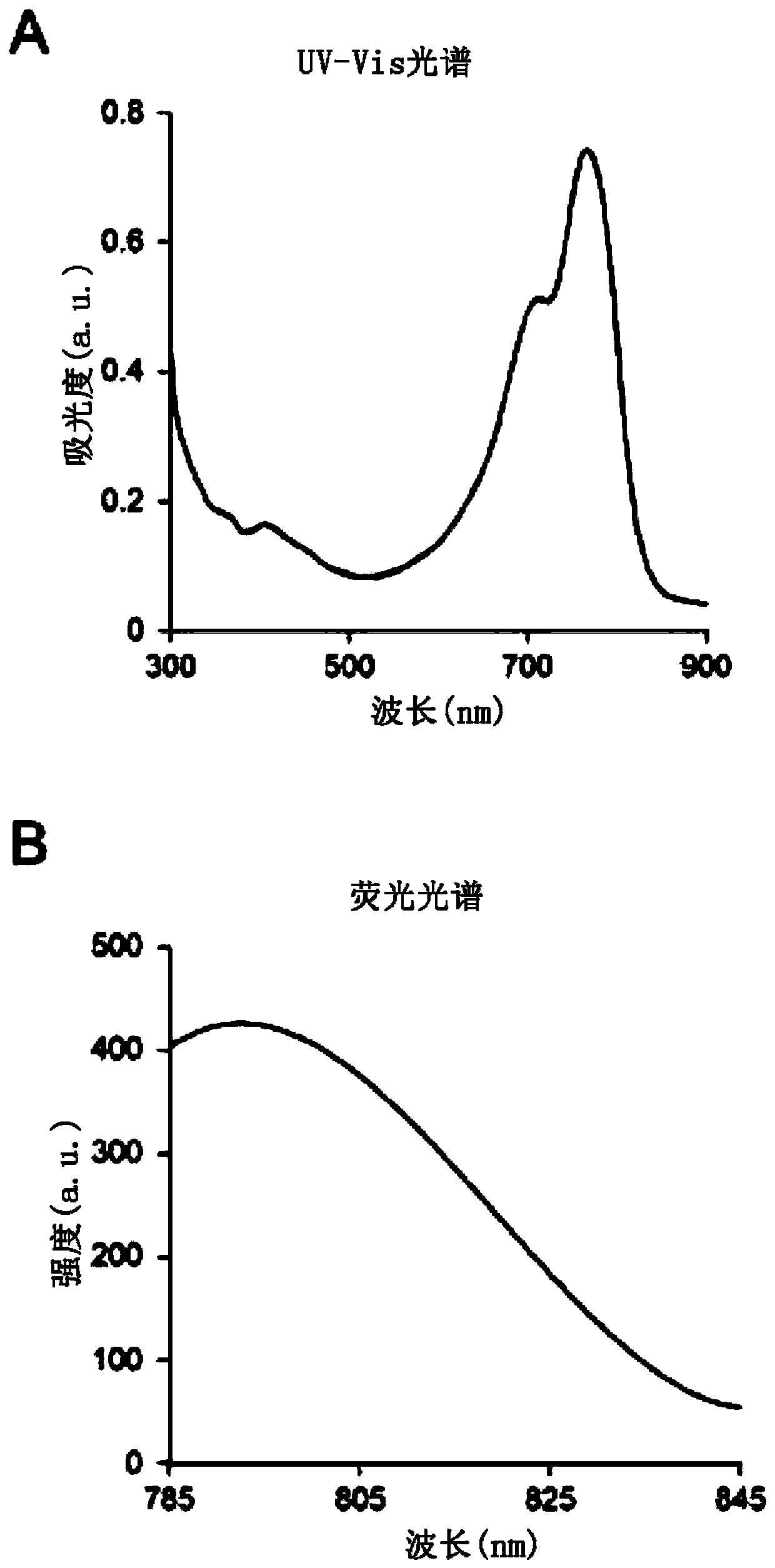
[0091] [Example 1] Preparation of Flamma774-fucoidan conjugate
[0092] The amine group of Flamma774, which is a near-infrared fluorescent dye of Bioacts, and the carboxyl group of fucoidan, are used to synthesize a covalent conjugate using a coupling agent. Flamma774-amine is a fluorescent substance with a substance mass of 971.15g / mol, a maximum excitation wavelength of 774nm, and a maximum release wavelength of 806nm. The near-infrared fluorescent dye conjugate has high permeability to biological tissues, so it can be used for biological imaging in drug delivery and tumor research. The fucoidan was produced by Sigma-Aldrich with a molecular weight of 18,000 Da extracted from Fucus.
[0093] Various coupling agents can be used to bind fluorescent dyes to fucoidan, where N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC) and N- Hydroxythiosuccinimide (thio-NHS) activates the dye to obtain a fucoidan-NHS ester conjugate, which is combined with Flaamma774-amine. T...
Embodiment 2
[0095] [Example 2] Preparation of ATTO 655-fucoidan conjugate
[0096] A coupling agent is used to form a covalent conjugate between the amine group of ATTO 655 as a fluorescent dye and the carboxyl group of fucoidan. ATTO 655-amine is a fluorescent substance with a substance mass of 798g / mol, a maximum excitation wavelength of 1663nm, and a maximum release wavelength of 680nm. The fucoidan was produced by Sigma-Aldrich with a molecular weight of 18,000 Da extracted from Fucus.
[0097] Use N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (thio-NHS) to activate fucoidan to obtain Fucoidan-NHS ester conjugate, combined with ATTO 655-amine. To explain the process in more detail, dissolve 10 mg of fucoidan in 0.1M 2-(N-morpholine)ethanesulfonic acid (MES) buffer, add 19.7 mg of EDC, 2.17 mg of thio-NHS, and stir for approximately 30 minutes. Then, after separation using a PD-10 column, 0.399 mg of ATTO655-amine fluorescence was added...
Embodiment 3
[0099] [Example 3] Preparation of ZW800-fucoidan conjugate
[0100] The amine group of the near-infrared fluorescent dye ZW800 developed by the research group of Professor Cui Xuezhu of Harvard University and the carboxyl group of fucoidan form a covalent conjugate using a coupling agent. ZW800-amine is a near-infrared fluorescent substance with a substance mass of 887g / mol, a maximum excitation wavelength of 753nm, and a maximum emission wavelength of 772nm. The fucoidan was produced by Sigma-Aldrich with a molecular weight of 18,000 Da extracted from Fucus.
[0101] Use N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (thio-NHS) to activate fucoidan to obtain Fucoidan-NHS ester conjugate, combined with ZW800-amine. The reaction ratios of fucoidan and ZW800 fluorescent dye are 1:2 and 1:4, respectively. If the process is described in more detail, 20mg fucoidan is dissolved in 0.1M 2-(N-morpholine)ethanesulfonic acid (MES) buffer, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com