Methods for the detection and quantification of circulating endothelial cells
A technology for circulating endothelial cells and nuclei, applied in the field of identification and quantification of circulating endothelial cells, can solve the problems of lack of detection sensitivity and specificity in CEC capture methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0089] Example 1. CEC identification and characterization using the HD-CEC assay
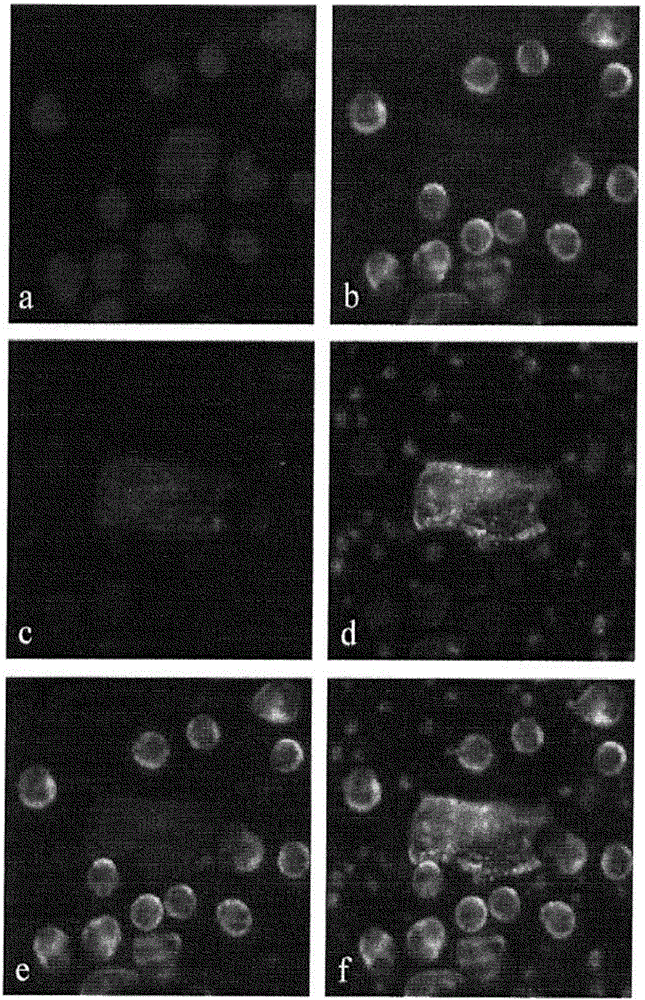
[0090] This experiment demonstrates that CEC cells can be characterized and identified using a combination of immunofluorescent markers and morphological features. The HD-CEC assay uses immunofluorescence and morphometric measurements to quantify, for example, the small number of CECs present in large numbers of WBCs in human blood.
[0091] Patient Selection and Blood Sample Collection
[0092] Recruited in 5 hospitals relatively close to the Scripps Research Institute Central Laboratory Scripps-Green Hospital, Scripps Mercy Hospital, Sharp Memorial, Sharp Grossmont and New Palomar Pomerado Hospital Patients recruited in this study. Institutional Review Board (IRB) approval was obtained from all recruiting networks, and all patients gave signed informed consent. Samples were collected from patients with suspected MI at the time of emergency room appearance and subsequently destroyed if the p...
example 2
[0104] Example 2. CEC counts of blood samples determined using HD-CEC
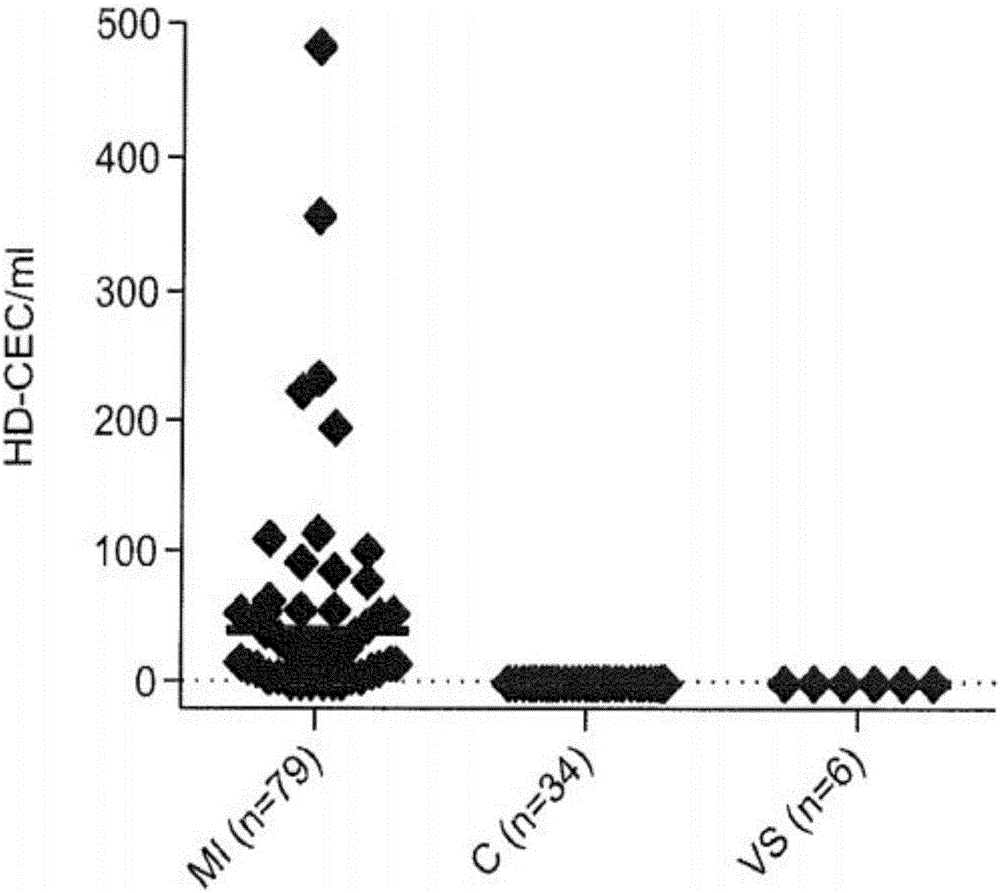
[0105] This experiment illustrates that the HD-CEC assay can be used quantitatively to obtain CEC counts in the blood of eg healthy humans and human patients (eg MI patients).
[0106] We collected and analyzed 79 peripheral blood draws from 79 MI patients; 28 blood samples were drawn into the Streck BCT and 51 into the EDTA BCT. We also collected a total of 34 blood samples from 25 healthy controls; 14 samples were drawn into the Streck BCT and 20 into the EDTA BCT (see Table 1).
[0107] Table 1. Results of CEC enumeration in blood samples of MI patients and healthy controls
[0108]
[0109] Sample size was broken down by study group and blood collection tube (BCT). Mean and median values and upper / lower quartiles are listed for each subject group in the study.
[0110] There were no significant differences in CEC counts detected in MI patients whose blood was drawn into EDTA and Streck tubes (P...
example 3
[0114] Example 3. Detection and Characterization of CEC Aggregates Using the HD-CEC Assay
[0115] This experiment exemplifies that the HD-CEC assay can detect CEC not only as single cells, but also in the form of CEC aggregates.
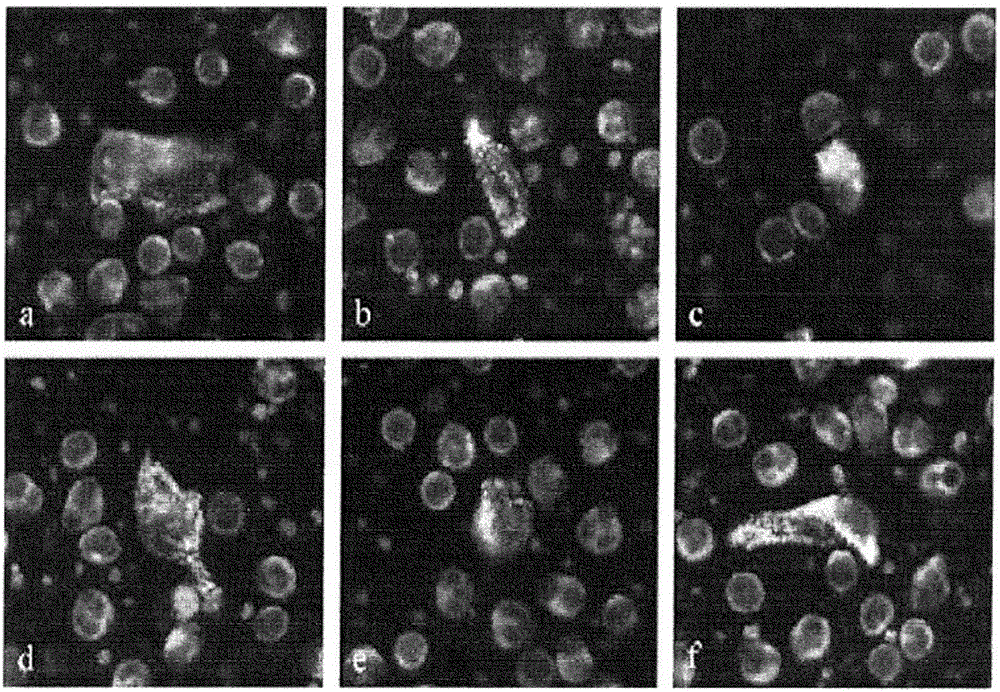
[0116] Not only CECs were found as single cells. CEC images of many MI patients often contain several nuclei (blue) associated with one cell body indicated by sequential double positive CD146 (red) and vWF (white) staining of the cytoplasm ( Figure 4 a) and b)). These images represent aggregates of damaged cells that are released into the circulation during plaque rupture.
[0117] Two or more CEC aggregates were detected in 65 of 79 MI patients; of these, 53 (81.5%) were found to have CEC aggregates, two or more cells exhibiting nuclear and / or cytoplasmic contacts . A measure of the percentage of CECs in aggregates (ie, total number of CECs in aggregates / total number of CECs) was found to be 46.3% ± 23.1% of CECs residing in aggregates in MI p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com