Oxadiazole-containing heterocyclic compounds, and preparation method and application thereof
A technology of compounds and heterocycles, applied in the field of oxadiazole-containing heterocycles and their preparation and application, can solve the problems of poor curative effect of leukemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
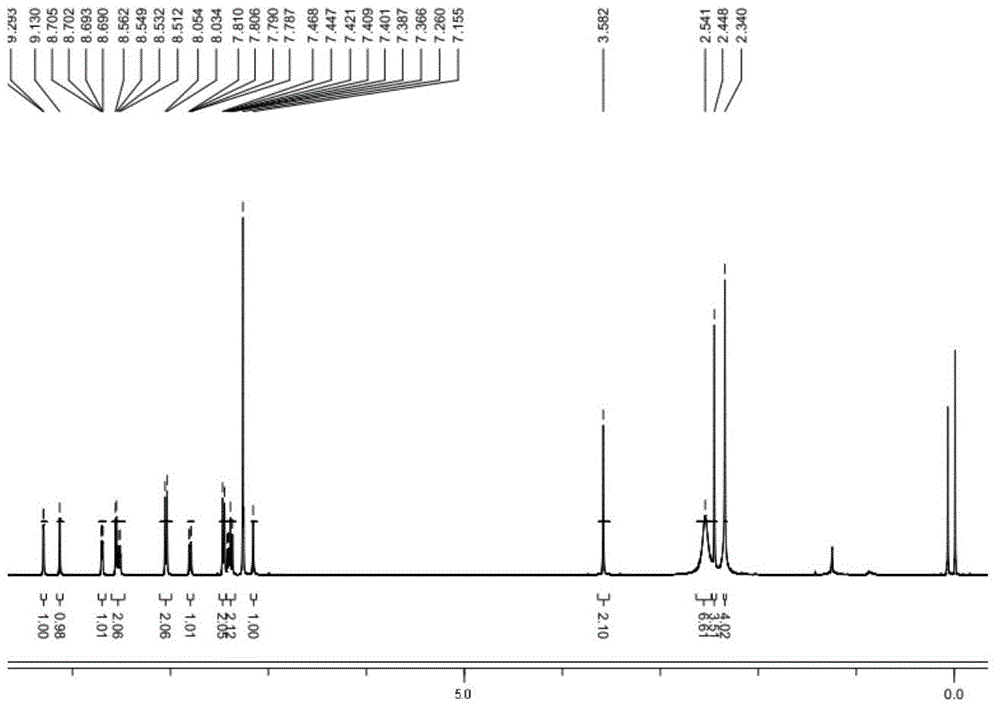
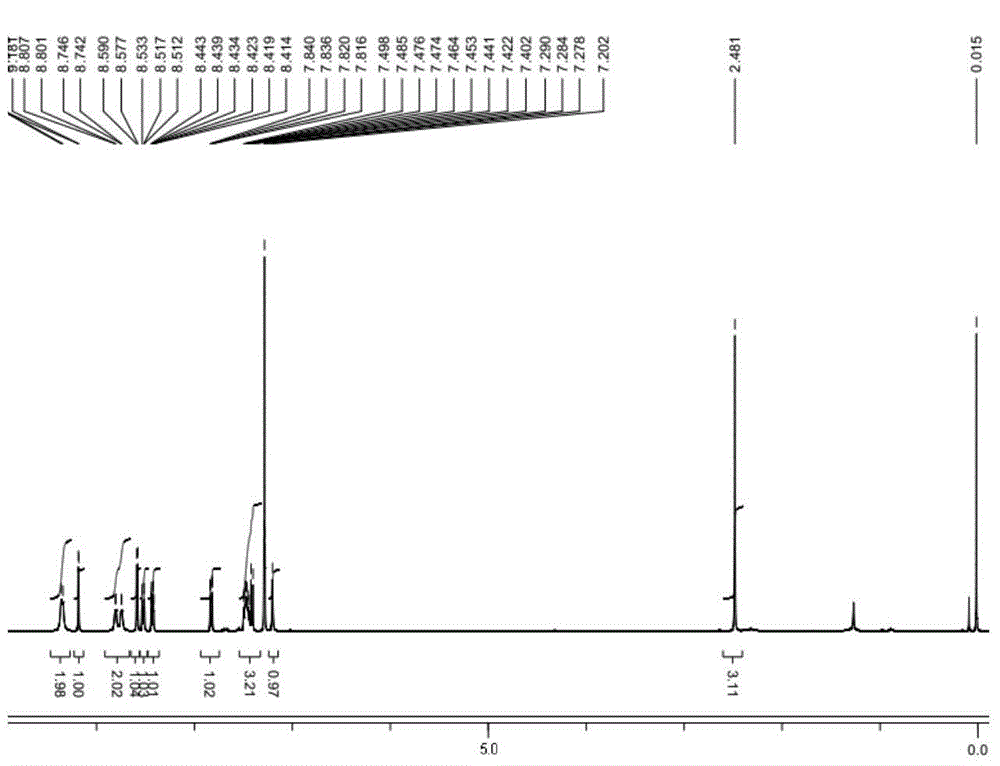
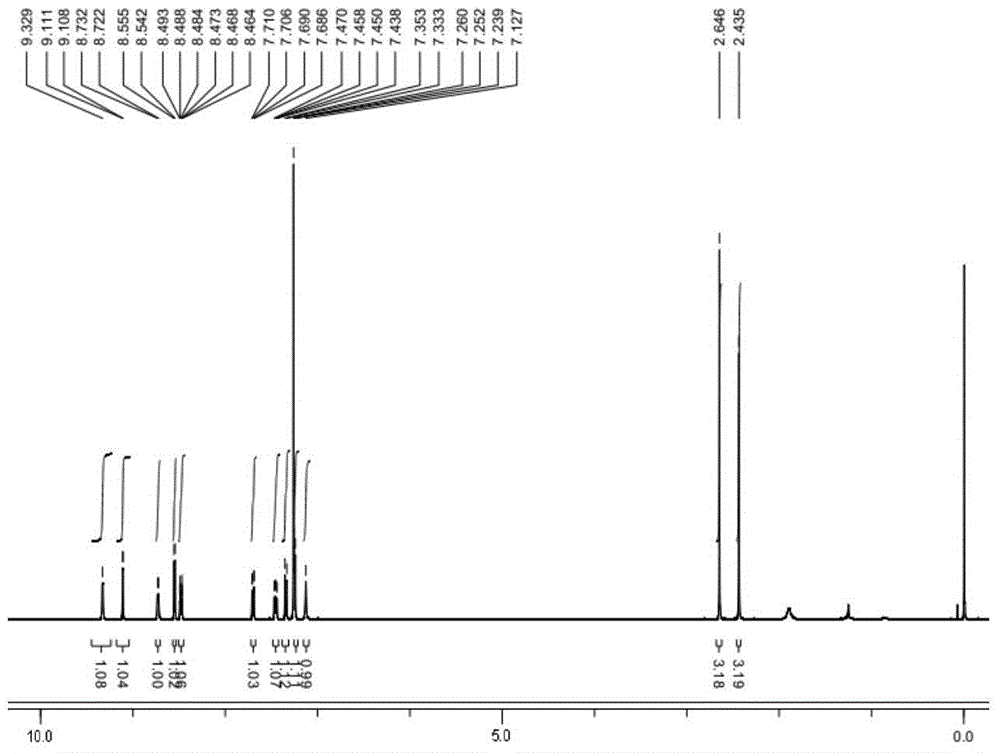
[0088] Example 1 Chemical synthesis and structural identification of oxadiazole-containing heterocyclic compounds provided by the present invention
[0089] 1. The oxadiazole-containing heterocyclic compound provided by the present invention has the following general structural formula:
[0090]
[0091] In the above general structural formula, the substituent -R includes the following groups:
[0092]
[0093] 2. The experimental procedures of chemical synthesis of heterocyclic compounds containing oxadiazole provided by the present invention:
[0094] 2.1 The general synthetic route is:
[0095]
[0096] 2.2.1 Synthesis of compound 5a-5h
[0097] 2.2.1.1 Compound 2: Synthesis of 4-methyl-3-[4-(pyridin-3-yl)pyrimidinyl-2-yl]aminobenzoic acid methyl ester
[0098]
[0099] Add 30mL of anhydrous methanol, compound 1 (1g, 3.26mmol) into a three-necked flask, slowly drop 2mL of concentrated sulfuric acid into it, after the addition is complete, the reaction solution is heated to reflux at 65...
example 1-8
[0142] Example 1-8 Synthesis of Compound 6a-6h
[0143]
example 1
[0144] Example 1 Compound 6a: N-(2-methyl-5-{5-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-1, Synthesis and structure identification of 3,4-oxadiazol-2-yl)phenyl)-4-(3-pyridyl)-pyrimidin-2-amine
[0145]
[0146] Add 167 mg of triphenoxy phosphine and 2 mL of dry dichloromethane to a three-necked flask, slowly add 50 μL of trifluoromethanesulfonic anhydride at 0°C, stir at 0°C for 5 min, and slowly rise to room temperature, a white solid is formed , Add compound 5a (114.5mg, 0.2mmol), the solution turns into bright yellow, stir the reaction at room temperature for 3h, then use 10% NaHCO 3 The aqueous solution was quenched, the aqueous layer was extracted with dichloromethane, and the dichloromethane layer with anhydrous Na 2 SO 4 Dry, filter, spin off the solvent from the filtrate, and purify the residue by column chromatography with CH 2 Cl 2 :MeOH=50:1,CH 2 Cl 2 : MeOH=30:1 gradient elution to obtain 65 mg of white solid, yield: 58.61%.
[0147] MS(ESI + ): 554.98; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com