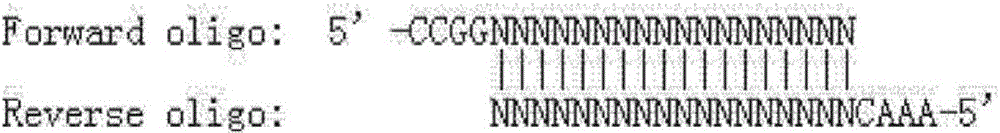Human-STAT6-targerted CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 system and application thereof in treating allergic diseases
A targeted technology for allergic diseases, applied in the field of genetic engineering, can solve problems such as low efficiency of the method, and achieve the effects of improving efficiency, saving costs, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The design of embodiment 1sgRNA
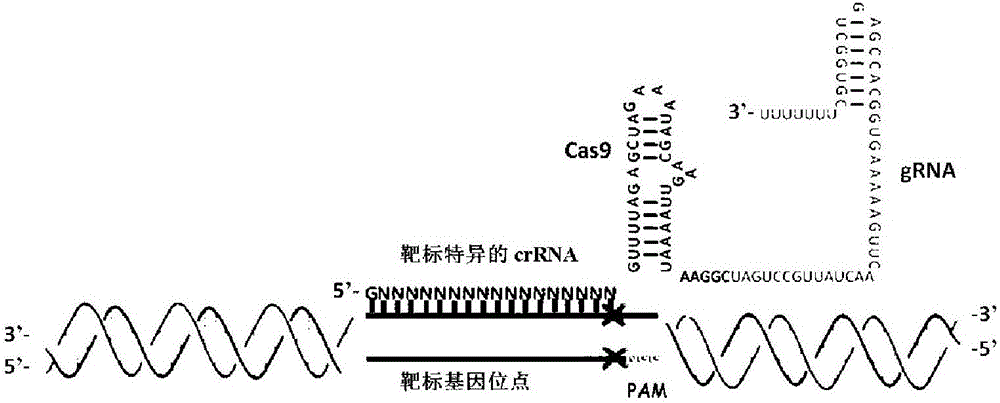
[0051] First, select the 5'-GGN(19)GG sequence based on the STAT6 gene shown in SEQ ID NO: 1. If there is no 5'-GGN(19)GG sequence, 5'-GN(20)GG or 5'- N(21)GG also works. The targeting site of the sgRNA on the STAT6 gene is located in the exon of the gene. Use BLAT in the UCSC database or BLAST in the NCBI database to determine whether the target sequence of the sgRNA is unique. At the same time, according to the design rules of other sgRNAs, a total of 97 sgRNAs were designed. However, the final experiment confirmed that only 15 of them had the function of targeted modification. Here, the sequences without functions are not listed one by one, only given 3 counter-examples, which also fully demonstrate that in the prior art, the design of sgRNA cannot obtain functional sgRNA only according to the design rules without experiments. The sgRNA sequence involved in the present invention is as follows:
[0052] STAT6-sg1: ggaagtgcccgctgaga...
Embodiment 2
[0070] Embodiment 2, construct the oligonucleotide duplex of sgRNA
[0071] According to the selected sgRNA: STAT6-sg1, add CCGG to its 5' to get a forward oligonucleotide (Forwardoligo) (if the sequence itself already has 1 or 2 Gs at the 5' end, then omit 1 or 2 accordingly G): According to the selected sgRNA, obtain the complementary strand of its corresponding DNA, and add AAAC to its 5' to obtain a reverse oligonucleotide (Reverseoligo). The above-mentioned forward oligonucleotide and reverse oligonucleotide were synthesized respectively. For STAT6-sg1: ggaagtgcccgctgagaaagg, the forward oligonucleotide (Forward oligo) designed was: CCggaagtgcccgctgagaaagg (SEQ ID NO: 20); reverse oligonucleotide The nucleotide (Reverse oligo) is aaaccctttctcagcgggcac (SEQ ID NO: 21).
[0072] The forward oligo and reverse oligo of the synthesized sgRNA oligonucleotide are denatured and annealed in pairs, and after annealing, a double strand that can be connected to the U6 eukaryotic exp...
Embodiment 3
[0075] Embodiment 3, the construction of sgRNA oligonucleotide plasmid
[0076] 1. Linearize the pGL3-U6-sgRNA plasmid. Enzyme digestion system and conditions are as follows: 2 μg pGL3-U6-sgRNA (400ng / μl); 1 μl CutSmart Buffer; 1 μl BsaI (NEB, R0535L); replenish water to 50 μl, incubate at 37 degrees for 3-4 hours, shake and centrifuge at intervals To prevent droplets from evaporating onto the tube cap.
[0077] 2. Ligate the annealed sgRNA oligonucleotide duplex to the linearized pGL3-U6-sgRNA plasmid to obtain the pGL3-U6-stat6-sg1 plasmid.
[0078] 3. Transform and coat Amp+ plates (50 micrograms / ml).
[0079] 4. Identify positive clones by sequencing with the universal primer U6, said primer being atggactatcatatgcttaccgta.
[0080] 5. Shake the bacteria overnight on a 37-degree shaker and extract the pGL3-U6-stat6-sg1 plasmid with a plasmid extraction kit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com