Pig PRRS purified vaccine and preparation method thereof
A technology for porcine PRRS and vaccines, applied in biochemical equipment and methods, recovery/purification, viruses, etc., can solve the problems of low virus content in vaccines, short cell maintenance time, and the impact of porcine PRRS immune prevention and control work, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
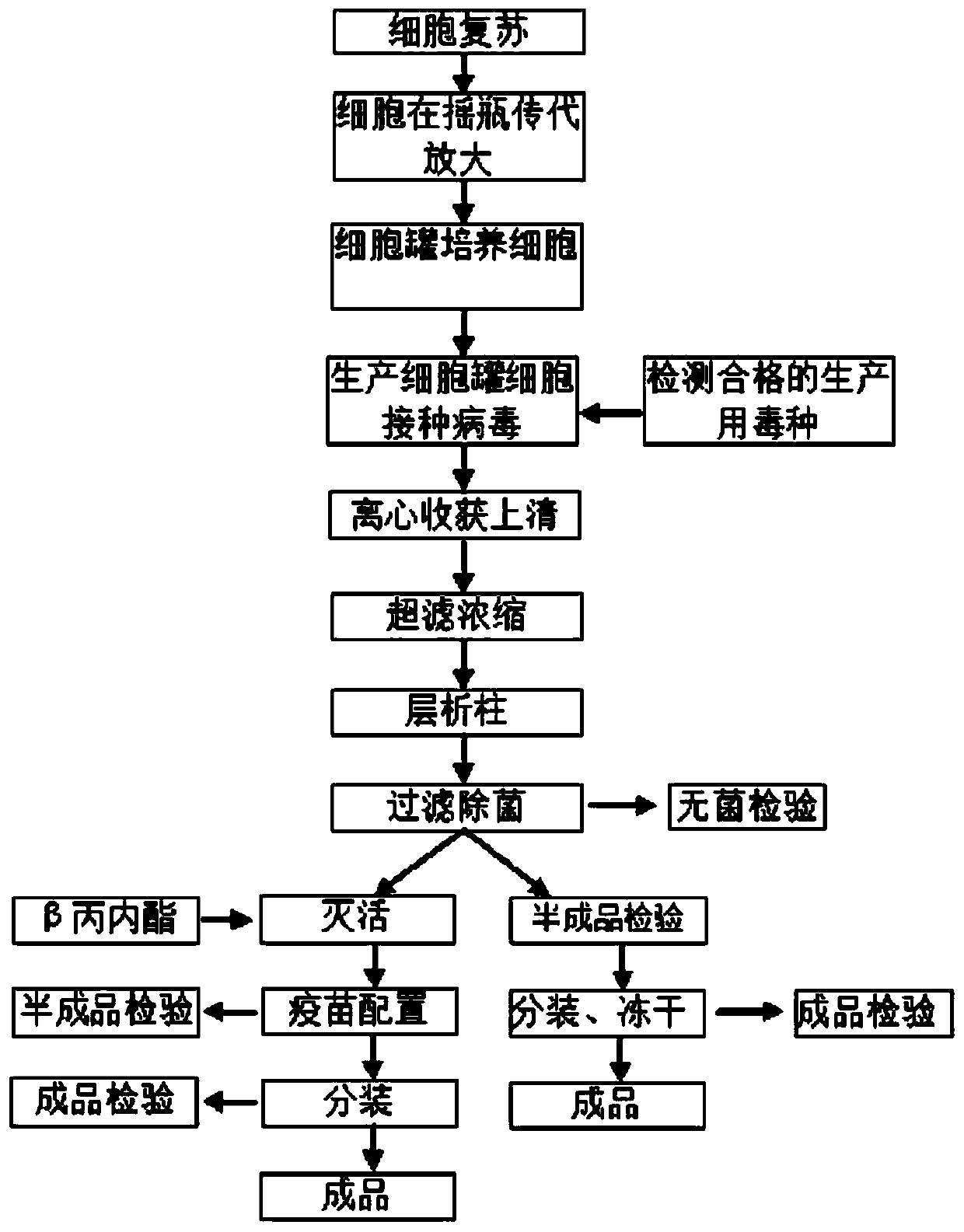
[0035] ——Full suspension culture of Marc-145 cells
[0036] Use shake flasks to resuscitate the cells frozen in liquid nitrogen, and when the cells grow to the required amount, inoculate the cell bioreactor for culture.
[0037] Specific steps are as follows:
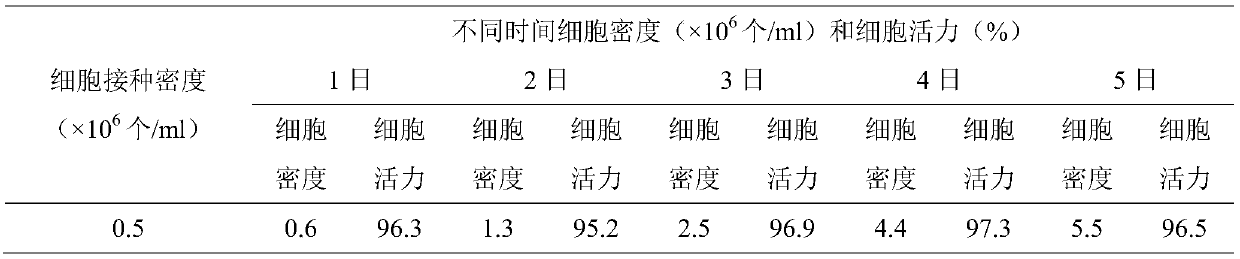
[0038] (1) The Marc145-QLS strain cells frozen in liquid nitrogen were taken and melted in a water bath at 37°C, then inoculated into the shake flask culture medium, and cultured at 37°C, with the rotation speed set at 120r / min. Cells were cultured for 3 to 4 days after recovery by 0.5×10 6 cells / ml seeding density for subculture expansion.
[0039] (2) Dilute the cells expanded in the above step (1) to 1.0×10 6 The bioreactor was inoculated at a density of 1 / ml, and the temperature was 27° C., pH 7.2, DO 50%, and the rotation speed was 120 r / min. The growth of the cells meets the requirements of cell proliferation, and is close to the proliferation speed, cell viability, and plateau phase of the cells cultured in s...
Embodiment 2
[0043] ——Marc145-QLS Cell Culture Attenuated PRRS Virus Strain
[0044] The cell density at the time of inoculation was 2.5×10 6 Individuals / ml, 0.1% inoculation of porcine reproductive and respiratory syndrome virus JXA1-R strain, when the cell viability is less than 70%, take a sample to determine the virus titer (TCID 50 / ml), the results are shown in Table 2.
[0045] Table 2 Virus titers inoculated with different cell densities
[0046]
Embodiment 3
[0048] ——Purification of PRRS virus cultured in Marc145-QLS cells
[0049] The harvested cell suspension is clarified or centrifuged by continuous flow to remove impurities such as cell debris. According to the size of the PRRS virus, an ultrafiltration membrane with a molecular cut-off of 100KD can be selected to concentrate the virus solution 50-100 times. The high-power concentrated solution is placed on a Separate6FF chromatography column, equilibrated with pH 7.0-7.4, 0.01M PBS buffer, and then subjected to column chromatography. The eluate is detected with a UV spectrophotometer A280nm, and the virus flows out. Collect and store at 4°C , to obtain purified virus liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com