A left ventricular volume reduction device
A left ventricle and apex technology, applied in heart valve, medical science, surgery, etc., can solve the problems of limited shock absorption performance of the base, increased device cost, fatigue fracture, etc., to achieve enhanced volume reduction effect, enhanced blocking effect, strong The effect of fatigue resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
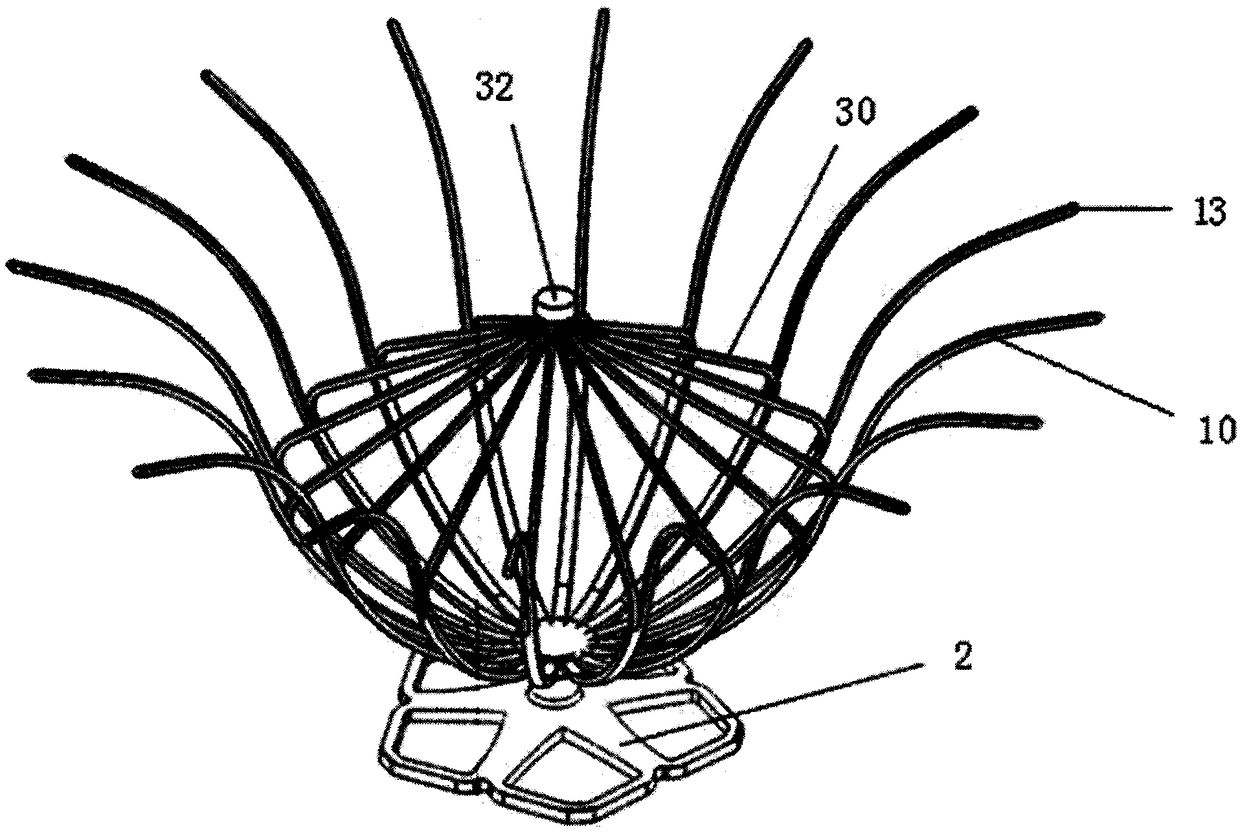
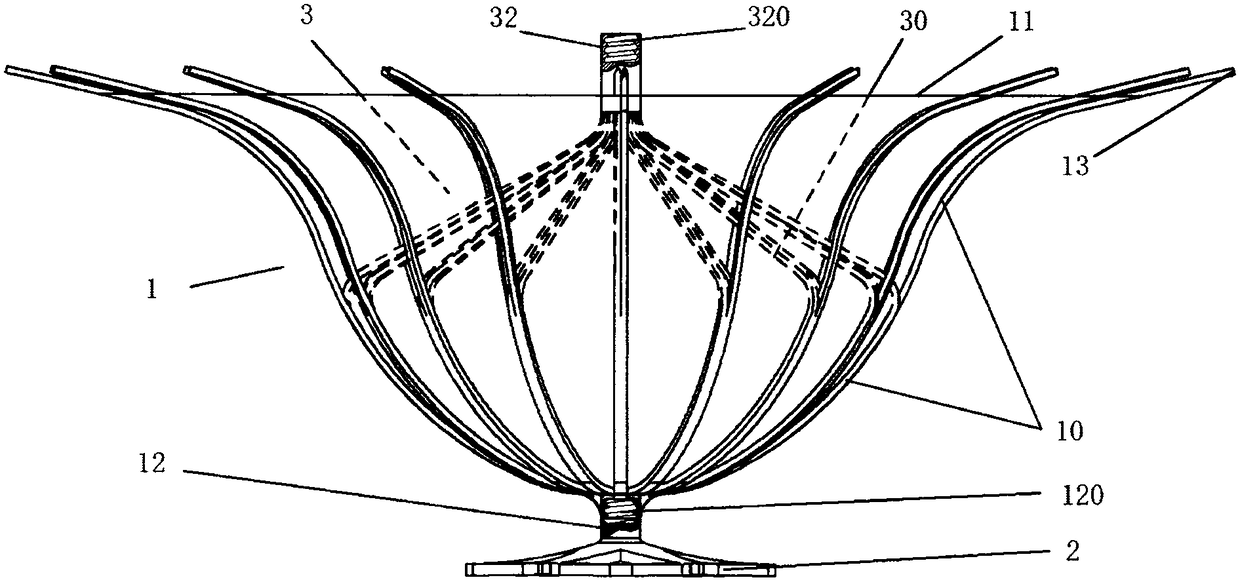
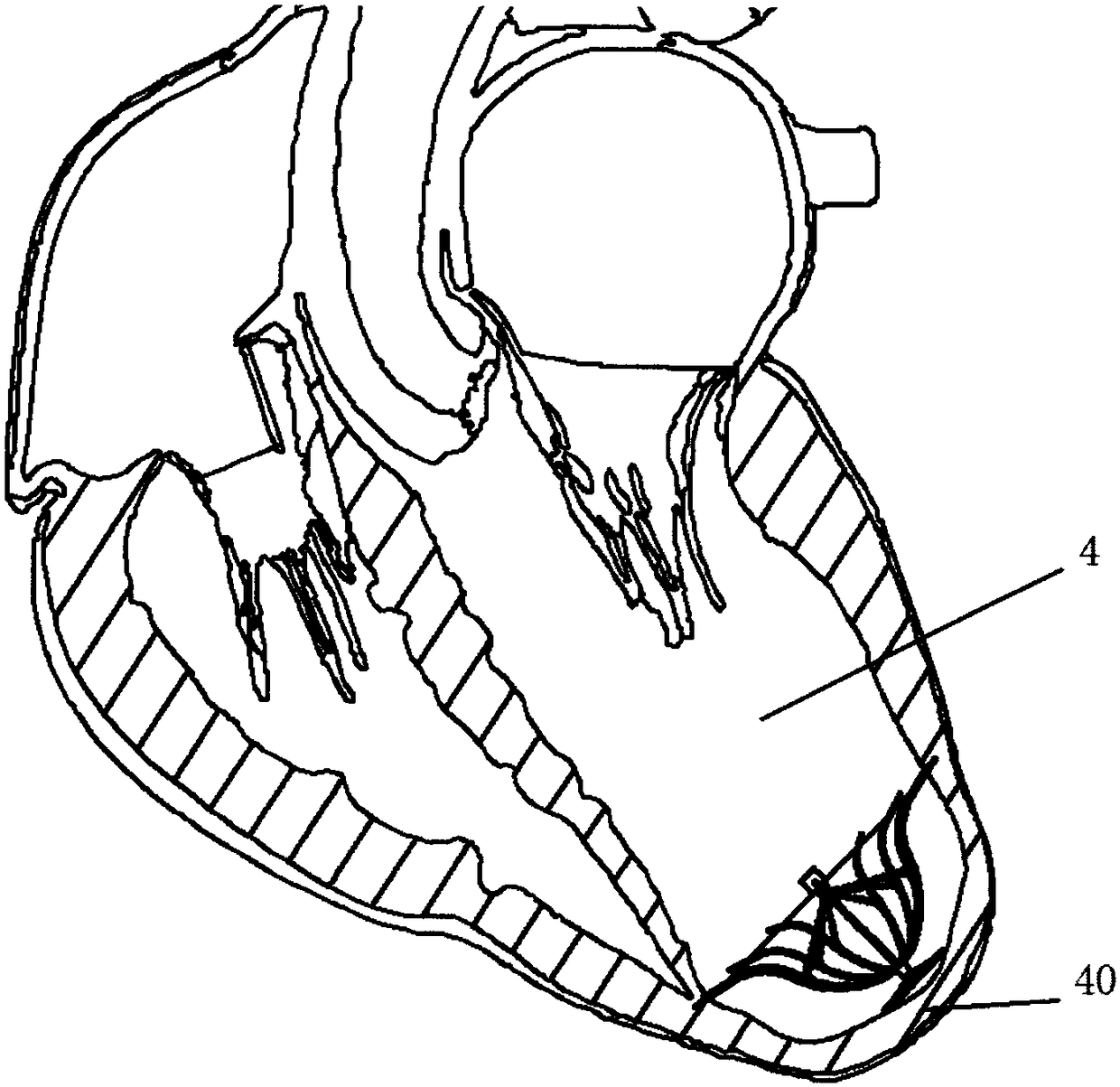
[0060] Such as figure 1 with 2 As shown, the left ventricular volume reduction device of the present invention includes a support frame 1 and a base 2 connected to the bottom 12 of the support frame 1. The support frame 1 is composed of a plurality of support rods 10, and the proximal ends of the support rods 10 are gathered to form a support frame 1, the distal end of the support rod 10 is adaptively opened in the direction of the distal end along the inner wall of the left ventricle 4, and the auxiliary umbrella 3 is also included in the space defined by the plurality of support rods 10, the auxiliary umbrella 3 is composed of a plurality of auxiliary umbrella ribs 30, the auxiliary ribs 30 extend from the central end 32 to the apex 40, and their terminals are connected to the middle of the support rods 10. Multiple auxiliary ribs 30 are in one-to-one correspondence with multiple support rods 10, and the surface of the support frame 1 is all Covered with a first film 11 . ...
Embodiment 2
[0067] Such as Figure 5 As shown, based on Embodiment 1, the difference between Embodiment 2 and Embodiment 1 is that the surface of the support frame 1 is only partially covered with the first film 11, that is, the support rod 10 and the auxiliary umbrella on the support frame 1 are covered. The connection point of the bone 30 extends to the distal area of the support frame 1, while the surface of the auxiliary umbrella 3 is entirely covered with the second membrane 31, and the proximal end of the first membrane 11 is in a sealed connection with the proximal end of the second membrane 31.
[0068] The optional membrane material of the second membrane 31 on the auxiliary umbrella 3 includes expanded polytetrafluoroethylene (ePTFE), polyester (PET), polyurethane elastomer (TPU), polyamide (PA), silica gel, degradable materials such as Polylactic acid (PLA), animal tissue, etc., after the device is released, due to the introduction of the second membrane 31, the device exhibi...
Embodiment 3
[0071] Such as Figure 7 As shown, based on Embodiment 1, the difference between Embodiment 3 and Embodiment 1 is that the proximal end of the auxiliary rib 30 of the auxiliary umbrella 3 is connected to the bottom 12, and the surface of the auxiliary umbrella 3 is completely covered with a second film 31. A plurality of auxiliary umbrella ribs 30 and the second polymer film 18 define a bladder 33, and the bladder 33 is preferably spherical or ellipsoidal. Here, the number of the auxiliary ribs 30 and the support rods 10 can be consistent or inconsistent. When the number of the auxiliary ribs 30 and the support rods 10 are inconsistent, only the auxiliary ribs 30 and the second membrane 31 can form a capsule 33. It is enough to increase the shock absorption effect and improve the durable life of the device, especially the bladder 33.
[0072] The proximal end of the auxiliary umbrella rib 30 is connected to the bottom 12 of the support frame 1, so that the device can support ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com