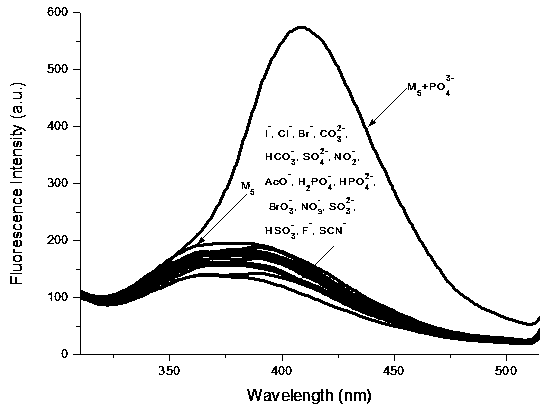
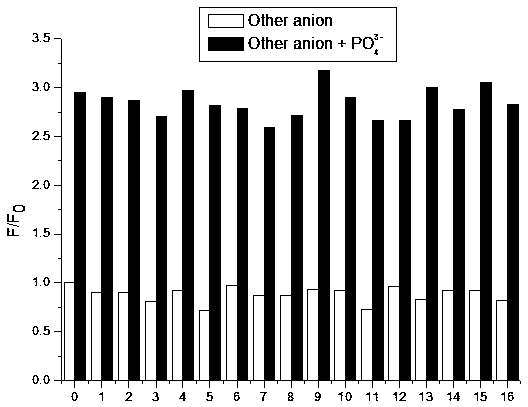
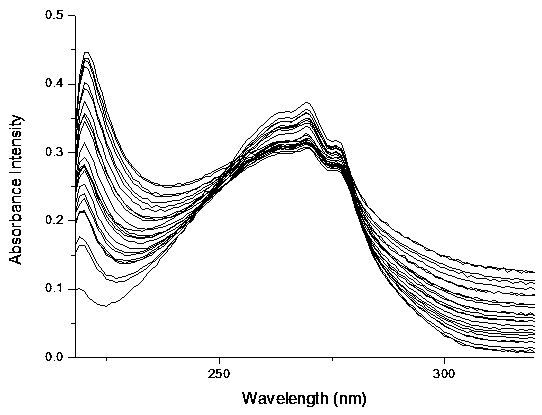
Synthesis and application method of benzimidazole derivative phosphate ion fluorescent probe
A phosphate ion and fluorescent probe technology, applied in the field of biochemistry, can solve the problems that cannot be directly identified independently, and achieve the effect of simple and fast detection operation, simple method, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment one: the benzimidazole derivative phosphate ion fluorescent probe 1,4-phenyl-bis[(carbamoylmethyl)-N-pentyl benzimidazolium ammonium chloride][ m 5 ] The structural formula is:
[0027] .
specific Embodiment approach 2
[0028] Specific embodiment two: the benzimidazole derivative phosphate ion fluorescent probe of specific embodiment one, 1,4-phenyl-bis[(carbamoylmethyl)-N-pentyl benzimidazolium ammonium chloride] [M 5 ] The synthetic method. Follow these steps:
[0029] 1. Weigh dichloroacetyl-p-phenylenediamine and N-pentylbenzimidazole with a molar ratio of 1: (2.1-2.8) and add it to a three-necked flask with a thermometer and a reflux condensing device. Add N, N -Dimethylformamide (DMF), the ratio of the amount of dichloroacetyl-p-phenylenediamine to the volume of DMF is 1mmol:(8mL-12mL), stirred until dissolved to obtain a reaction mixture;
[0030] 2. Raise the temperature of the reaction mixture to 110-130°C, follow the reaction by TLC, and finish the reaction for 3-6 hours, cool to room temperature, and filter to obtain the crude product;
[0031] 3. Mix methanol and ethyl acetate at a volume ratio of 1:(8-12), recrystallize the crude product three times with a mixed solvent, dry t...
specific Embodiment approach 3
[0032] Specific embodiment three: the difference between this embodiment and specific embodiment two is that the molar ratio of dichloroacetyl-p-phenylenediamine and N-pentylbenzimidazole in step one is 1:2.5, and dichloroacetyl-p-phenylenediamine The ratio of the amount of the substance to the volume of DMF is 1mmol:10mL; others are the same as in Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com