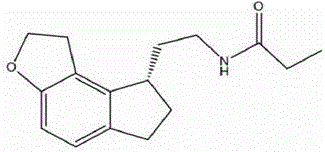
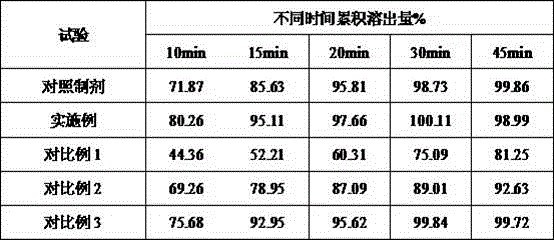
Orally disintegrating tablet containing ramelteon and preparation method of orally disintegrating tablet
A technology of ramelteon and orally disintegrating tablets is applied in the field of preparation of co-powder and orally disintegrating tablets, and can solve the problems of affecting the disintegration of orally disintegrating tablets, complicated process, time-consuming and labor-intensive and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0014] This embodiment is in the form of a tablet. (measured per 1000 pieces)
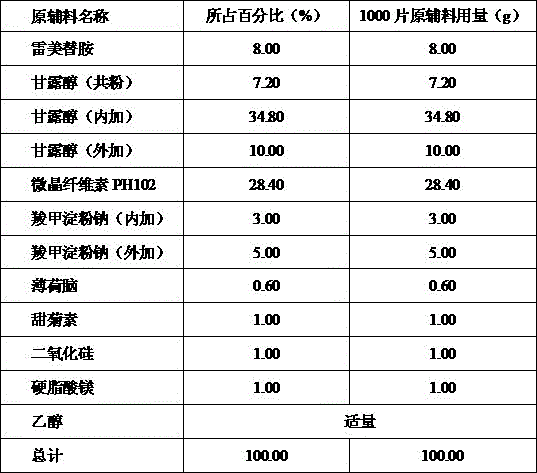
[0015] Name of raw material Percentage (%) Amount of raw materials and auxiliary materials for 1000 tablets (g) Ramelteon 8.00 8.00 Mannitol (co-powder) 7.20 7.20 Mannitol 44.80 44.80 Microcrystalline Cellulose PH101 28.40 28.40 Carboxymethyl Starch Sodium 2.00 2.00 Croscarmellose Sodium 6.00 6.00 Menthol 0.60 0.60 stevia 1.00 1.00 silica 1.00 1.00 Magnesium stearate 1.00 1.00 total 100.00 100.00
[0016] Preparation process: the active ingredient ramelteon in the prescription is mixed with the prescription amount of co-powder mannitol, and then micronized to obtain the blend, which is ready for use. Grind the remaining mannitol in the prescribed amount through a 100-mesh sieve for use. Mix co-powder, microcrystalline cellulose PH101, sodium carboxymethyl starch, croscarmellose sodium, menthol, stevia, si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com