Pre-filled plastic syringe containing VEGF antagonist
A syringe, pre-filled technology, applied in the directions of syringes, hypodermic injection devices, medical preparations containing active ingredients, etc., can solve problems such as unclear syringes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
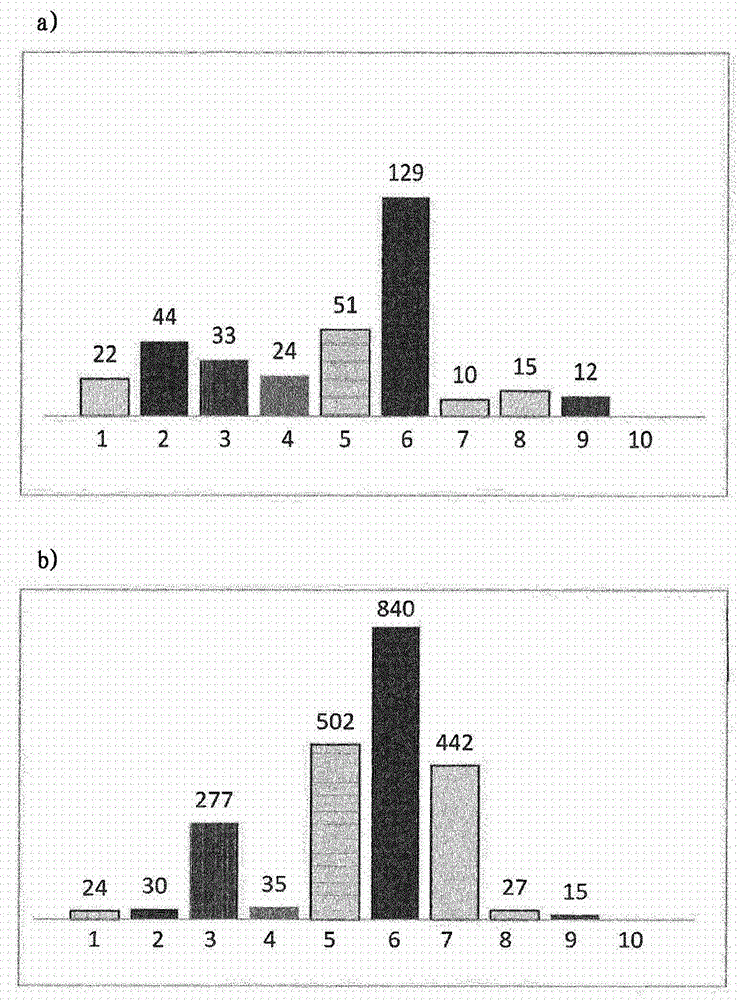
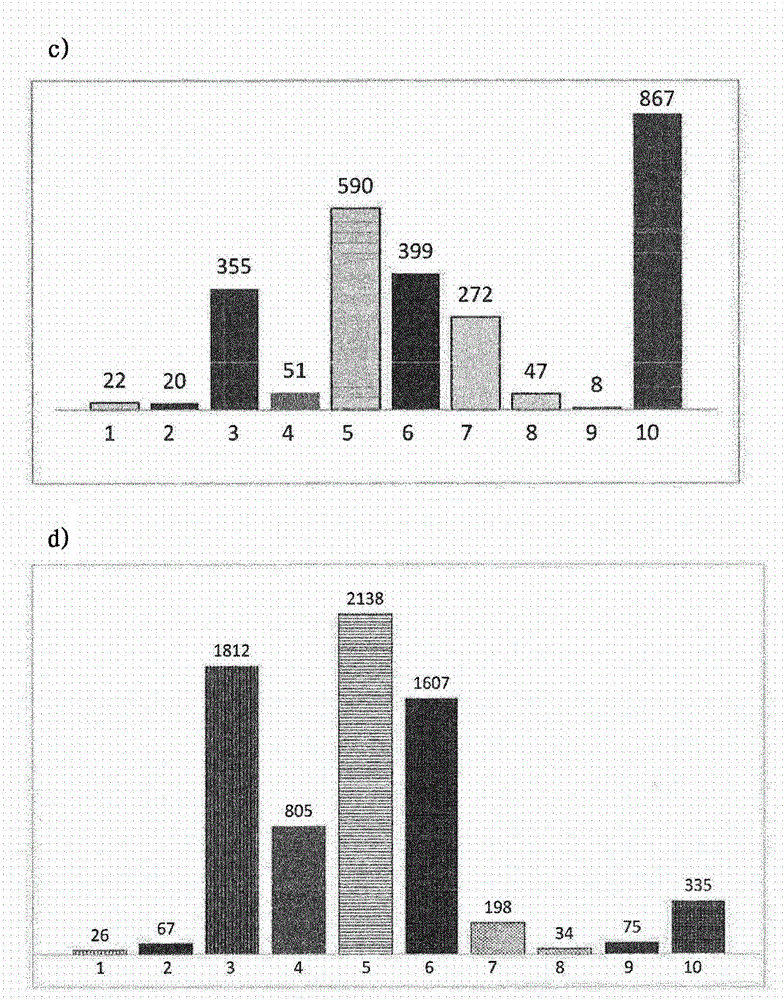
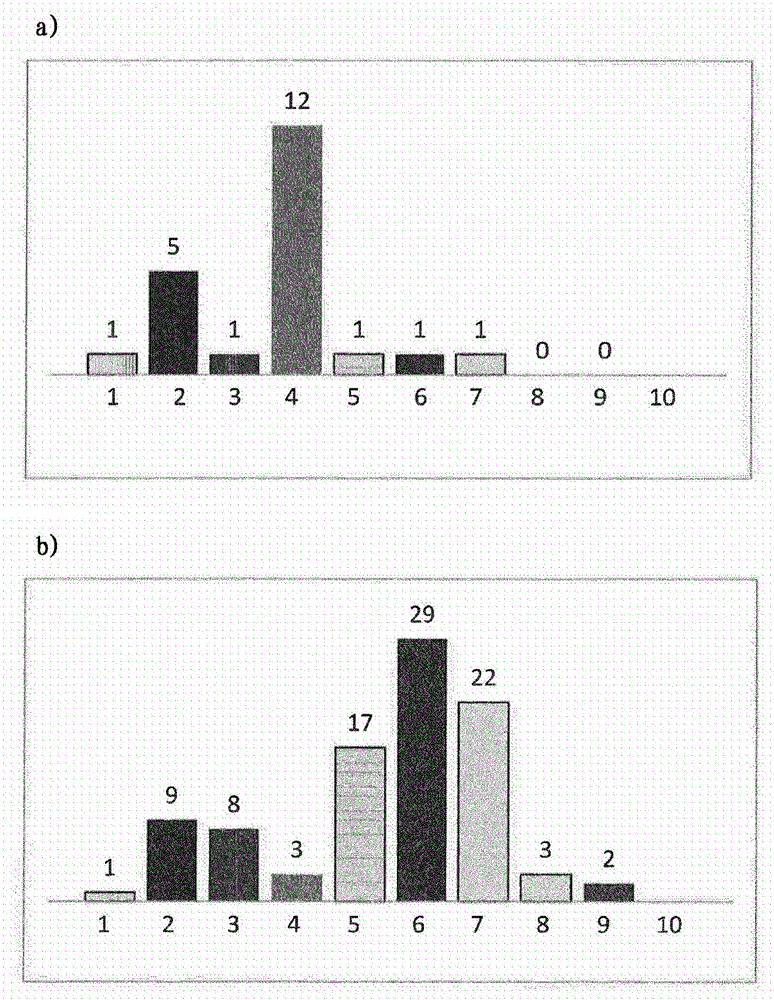
[0092] 1. Different sized syringes were assayed in different plastic and glass syringes containing ranibizumab and subjected to different conditions. particles
[0093] Fill 400 μl of a solution of anti-VEGF antibody ranibizumab containing 1 mg / ml antibody and histidine buffer, trehalose dihydrate, polysorbate 20, pH 5.5 into the following syringe:
[0094] Table 1:
[0095]
[0096] Syringes from Table 1 were rotated from needle to stopper at 1 cycle / 10 sec at 40°C for 5 minutes, two weeks and four weeks, or subjected to five freeze / thaw cycles (from +5 to -20°C). Light obscuration was then measured with a FlowCam PV benchtop system (FluidImaging Technologies Inc., Maine, USA) using the system software (VisualSpreadsheet software, version 3.4.8) and the following parameters:
[0097]
[0098] The results of the analysis are shown in figure 1 and figure 2 middle. The prefilled silicone-free cycloolefin polymer syringes 1 and 8 had low particle levels under all ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com