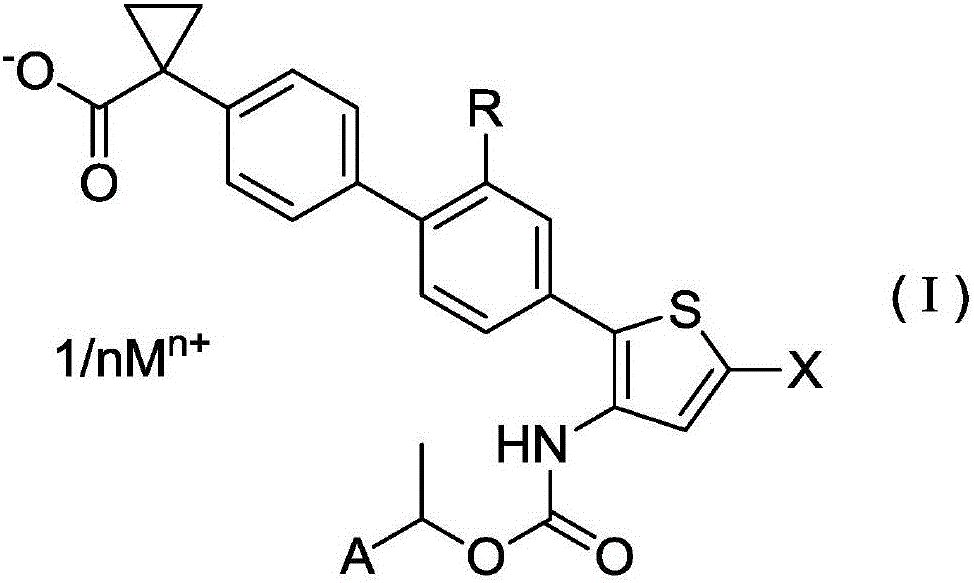
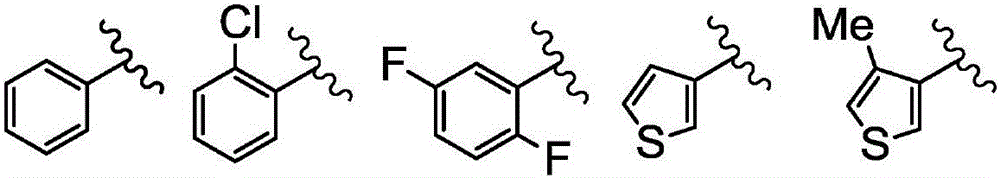
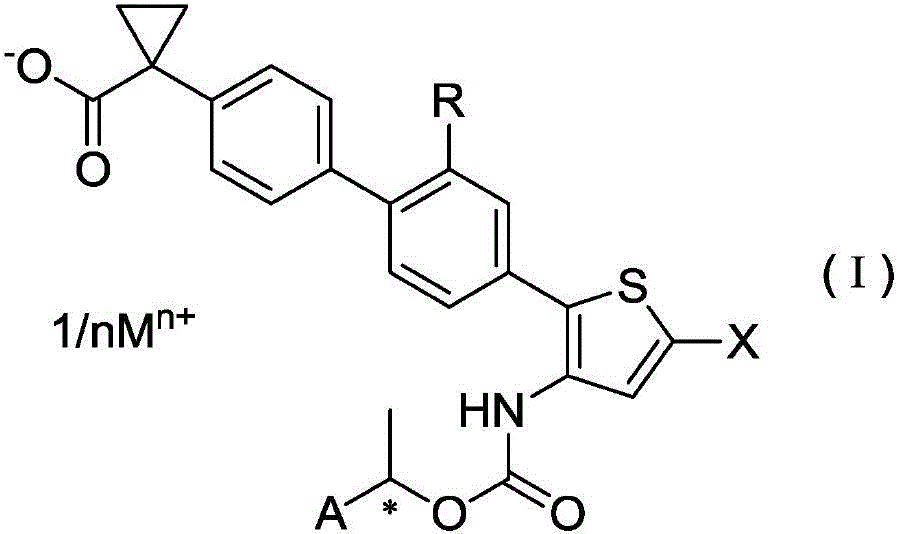
Salt of halogen-substituted heterocyclic compound
A halogen atom, cyclopropane carboxylic acid technology, applied in the direction of drug combination, organic chemistry, digestive system, etc., can solve the problem of undisclosed compounds and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] (R)-1-[4'-(5-chloro-3-{[(1-phenylethoxy)carbonyl]amino}thiophen-2-yl)-2'-methoxy-[1,1 Sodium '-biphenyl]-4-yl]cyclopropanecarboxylate (compound number I-2)
[0222]
[0223] (R)-1-[4'-(5-chloro-3-{[(1-phenylethoxy)carbonyl]amino}thiophen-2-yl)-2 synthesized in the same manner as in Reference Example 29 '-Methoxy-[1,1'-biphenyl]-4-yl]cyclopropanecarboxylic acid 1.10g (2.00mmol) in acetonitrile (80ml) suspension solution, while stirring under ice bath, add 1N After adding 2.00 ml (2.00 mmol) of an aqueous sodium hydroxide solution, ultrapure water (6 ml) was added, and ultrasonic treatment was performed to obtain a homogeneous solution, followed by stirring at room temperature for 3 hours. A small amount of ultrapure water was further added to the reaction mixture, freeze-dried, and then heated and dried under reduced pressure to obtain 1.08 g (1.89 mmol, yield 95%) of the target compound as a white solid.
[0224] Mass spectrometry (ESI + , m / z): 570[M+1] + .
[...
Embodiment 2
[0227] (R)-1-[4'-(5-chloro-3-{[(1-phenylethoxy)carbonyl]amino}thiophen-2-yl)-2'-methoxy-[1,1 Potassium '-biphenyl]-4-yl]cyclopropanecarboxylate (compound number I-4)
[0228]
[0229] (R)-1-[4'-(5-chloro-3-{[(1-phenylethoxy)carbonyl]amino}thiophen-2-yl)-2 synthesized in the same manner as in Reference Example 29 '-Methoxy-[1,1'-biphenyl]-4-yl]cyclopropanecarboxylic acid 275mg (0.501mmol) in acetonitrile (20ml)-ultrapure water (1.5ml) suspension solution, while stirring 0.500 ml (0.500 mmol) of 1N aqueous potassium hydroxide solution was added to make a uniform solution, and ultrapure water (6 ml) was added thereto, followed by ultrasonic treatment. After allowing the reaction mixture to stand at room temperature for 30 minutes, a small amount of ultrapure water was added, freeze-dried, and then heated and dried under reduced pressure to obtain 235 mg (0.401 mmol, yield 80%) of the target compound as a white solid.
[0230] Mass spectrometry (ESI + , m / z): 586[M+1] + . ...
Embodiment 3
[0233] (R)-1-[4'-(5-chloro-3-{[(1-phenylethoxy)carbonyl]amino}thiophen-2-yl)-2'-methoxy-[1,1 '-Biphenyl]-4-yl]cyclopropanecarboxylic acid 1 / 2 calcium (compound number I-6)
[0234]
[0235] (R)-1-[4'-(5-chloro-3-{[(1-phenylethoxy)carbonyl]amino}thiophen-2-yl)-2'-methanol obtained in Example 1 Oxy-[1,1'-biphenyl]-4-yl]cyclopropanecarboxylate sodium 101mg (0.177mmol) in ultrapure water (25ml) solution, after adding 0.5M calcium acetate aqueous solution 0.180ml (0.090mmol) , stirred at room temperature for 2 days. The obtained suspension solution was filtered with a membrane filter (manufactured by Millipore Corporation), washed with ultrapure water, and then heated and dried under reduced pressure to obtain 40.4 mg (0.071 mmol, yield 40%) of the target compound as a white solid. .
[0236] Mass spectrometry (ESI + , m / z): 1133[2M+1] + .
[0237] 1 H-NMR spectrum (400MHz, DMSO-d 6 )δ: 9.42 (1H, brs), 7.41-7.25 (10H, m), 7.19-7.15 (2H, m), 7.07 (1H, dd, J=7.9, 1.3Hz), 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com