Camelid single-domain antibody directed against phosphorylated TAU proteins and methods for producing conjugates thereof
A phosphorylation, domain technology, used in chemical instruments and methods, immunoglobulins, biomaterial analysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0234] Example 1: Generation of conjugation to ALEXA anti-phosphorylated TAU VHH and its body In vitro / in vivo evaluation
[0235] Materials and methods
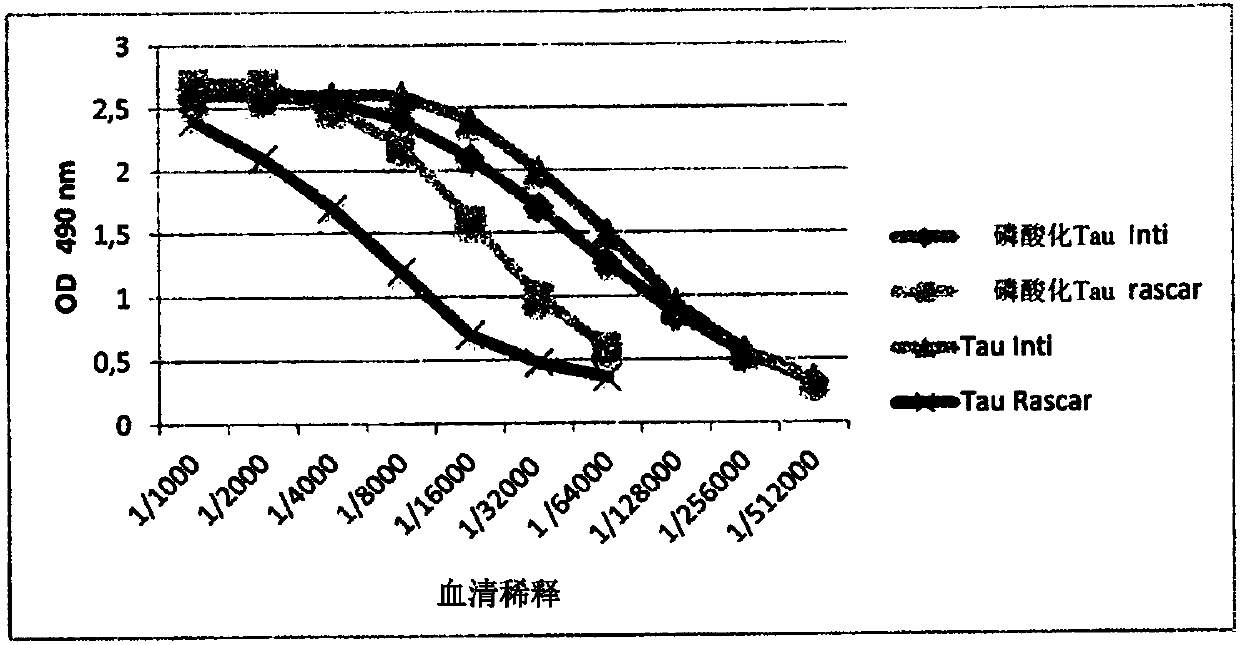
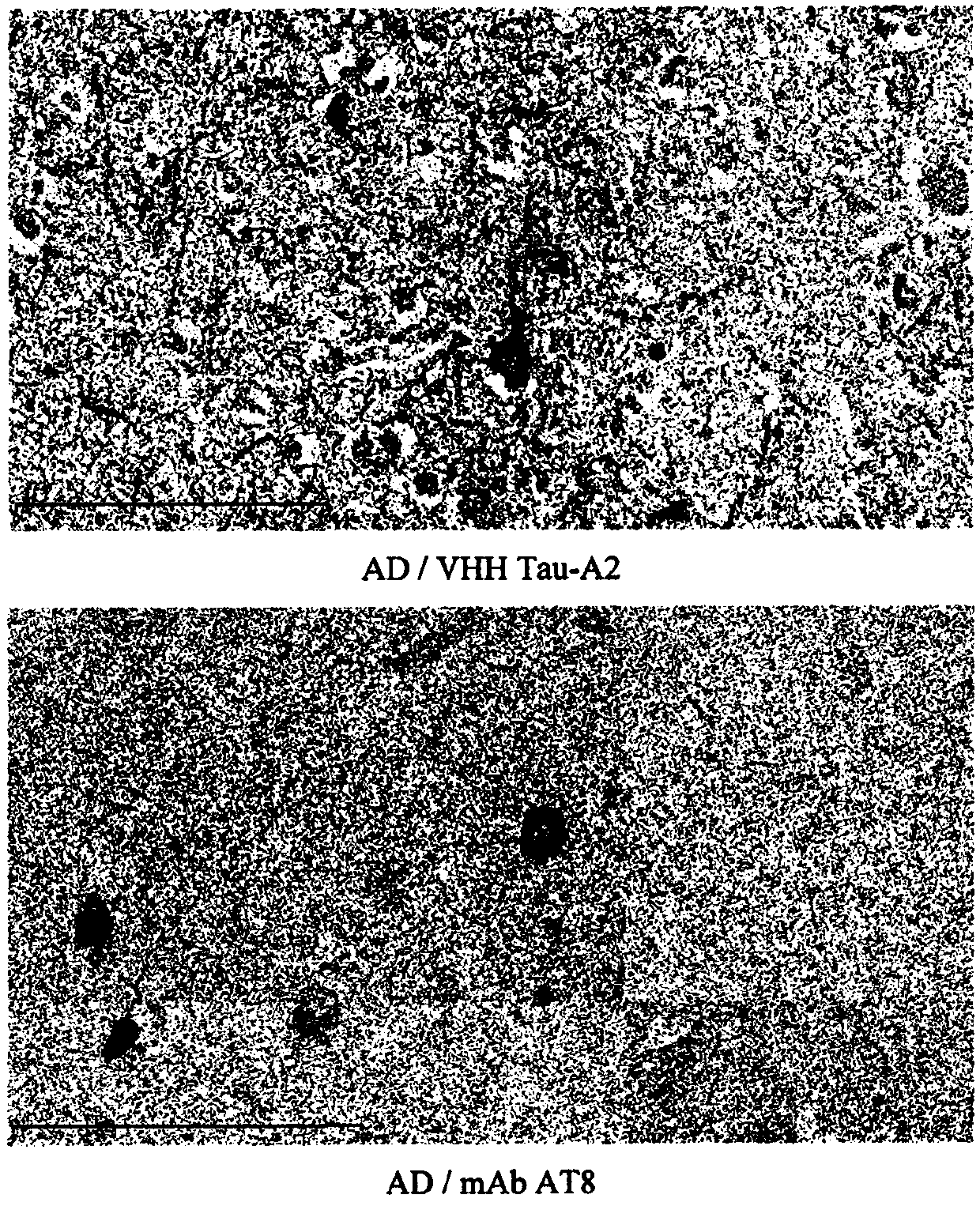
[0236] 1. Production, selection and purification of anti-tau-specific VHH (Tau-A2)
[0237] 1.1 Preparation of antigen and induction of humoral immune response in alpaca
[0238] subjects
[0239] Human cortical brain tissues from AD patients (Braak stages V and VI) were obtained from the NeuroCEB Brain Bank. The bank is linked to the Brain Donation Program, run by the consortium of patients associations (including the French Alzheimer's Association) and declared by the French Ministry of Research and Universities as required by French law . Express written consent was obtained for brain donation in accordance with French bioethics law.
[0240] Tissue extraction was performed according to Mercken et al. (1992 Acta Neuropathol., 84, 265-272). Cortex from AD brains (0.2 g) was homogenized in 10 volumes of ...
Embodiment II
[0328] Example II: Synthesis of a variant of TAU-A2-SH: TAU-A2VAR-SH
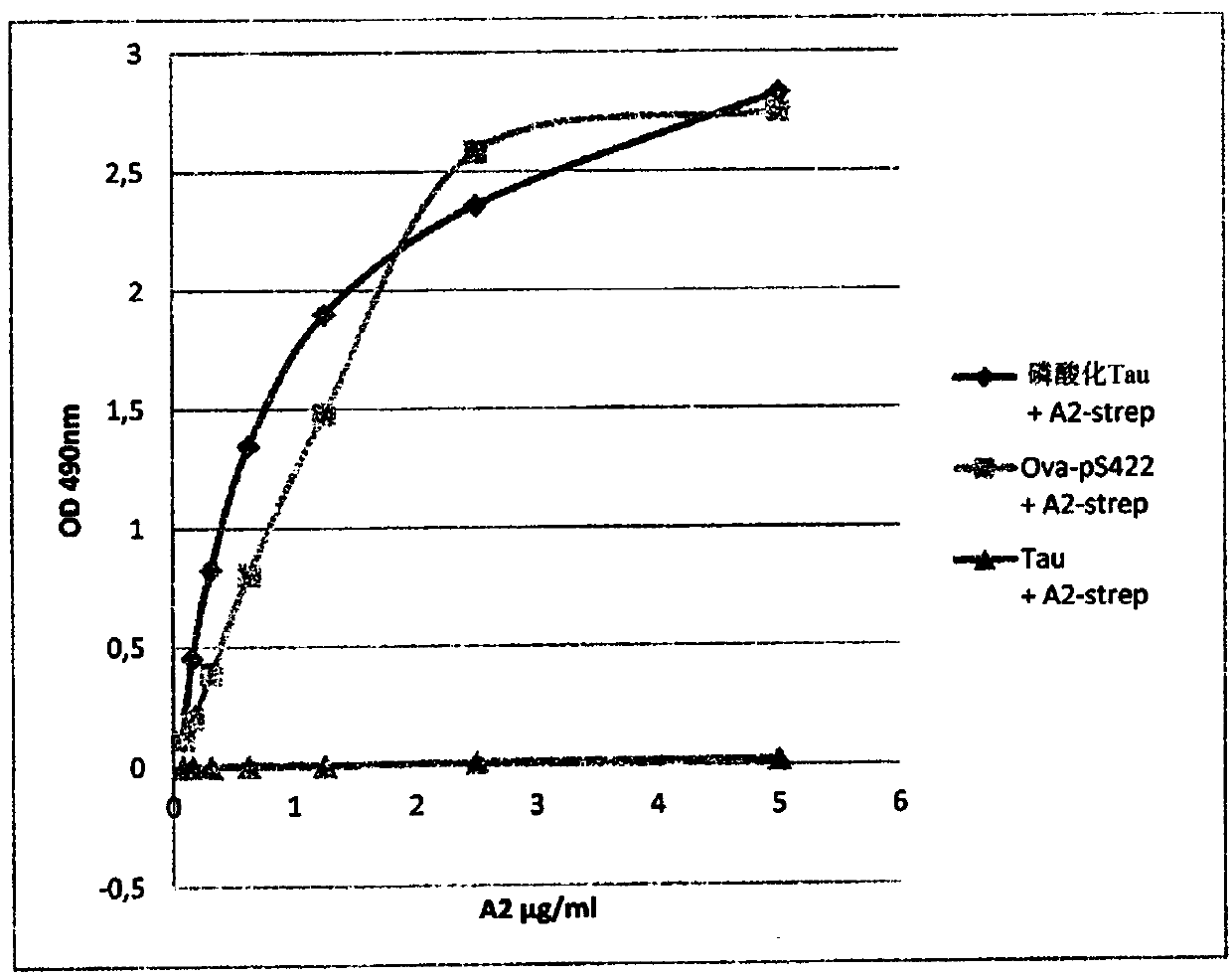
[0329] VHH Tau-A2 specifically detects phosphorylated Tau, but its production level is rather low, averaging 150 μg / L, and tends to aggregate. It is therefore desirable to provide a variant of VHH Tau-A2 with improved properties.
[0330] The 2 hydrophobic amino acids located in the 2 hydrophobic regions of VHH Tau-A2-SH shown in SEQ ID NO.14 are mutated: isoleucine at position 76 is replaced by glycine (I76G), and vale at position 110 Amino acid is replaced by glycine (V110G) ( Figure 10 ). These mutations increase the hydrophilicity of the VHH, with an increased GRAVY index ranging from -0.280 for Tau-A2-SH to -0.365 for the variants (GRAVY or Grand average hydrophilicity index indicates the solubility of the protein: positive GRAVY (hydrophobic ), negative GRAVY (hydrophilic)). Glutamine at position 26 was also mutated to glutamic acid (Q26E), and aspartic acid (D) and valine (V) were added betwee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com