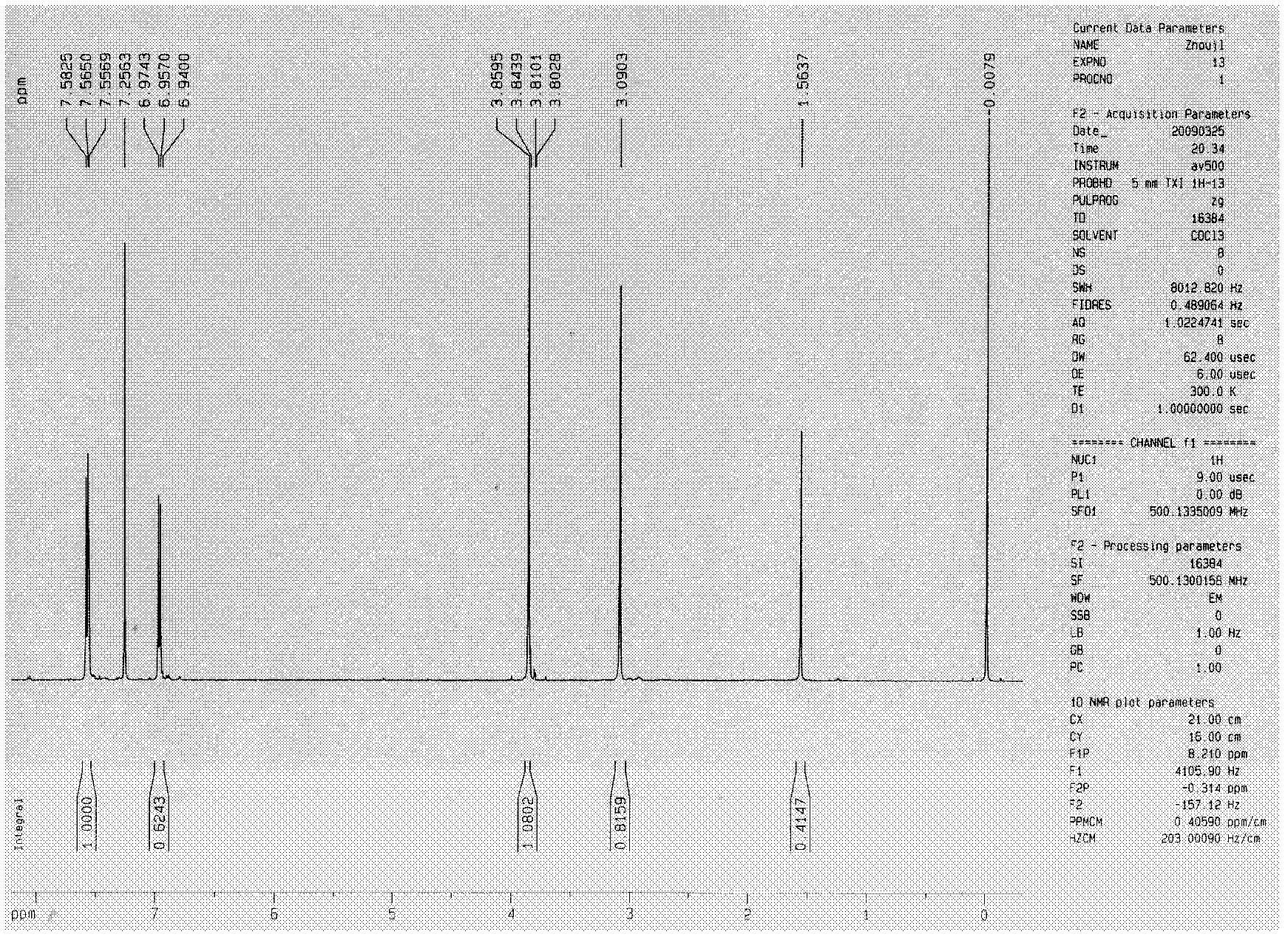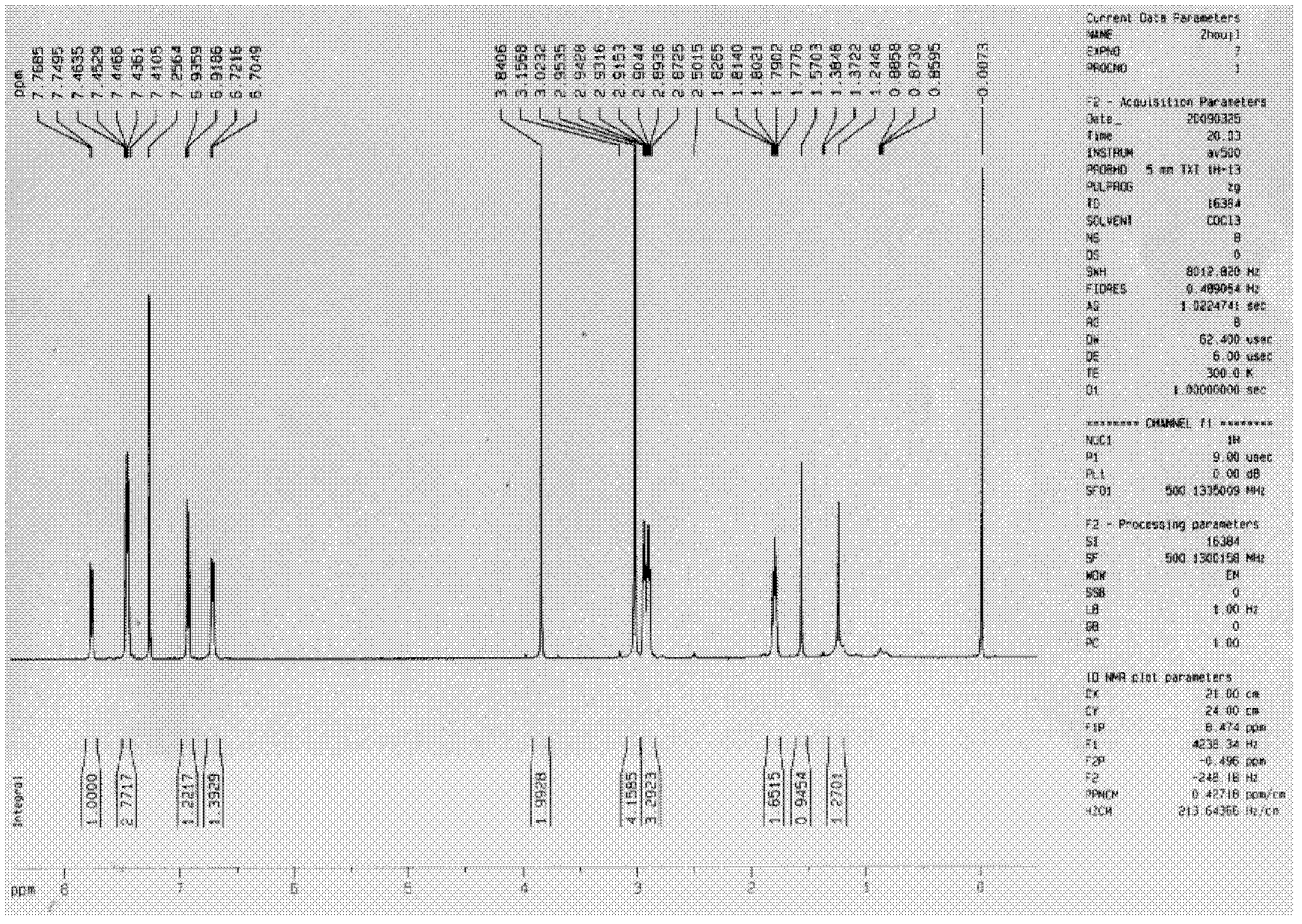Cyclic ketone derivatives and applications thereof as developers and aggregation inhibitors of amyloid protein sediments and neurofibrillary tangles
A technology of derivatives and cyclic ketones, which is applied in the field of preparation of labeled compounds, can solve the problems of large trauma, risk and slow clearance of biopsy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0321] Embodiment 1, the synthetic method of symmetrical cyclic ketone derivatives:
[0322]
[0323] Synthesis method of symmetrical cyclic ketone derivatives: Under the catalysis of sodium hydroxide solution, carry out Aldol condensation dehydration of cyclopentanone or cyclohexanone and corresponding benzaldehyde to obtain the target compound.
Embodiment 2
[0324] Embodiment 2, the synthetic method of asymmetric cyclic ketone derivatives:
[0325]
[0326] The synthesis method of asymmetric cyclic ketone derivatives: firstly, react with different substituted benzaldehydes and excess cyclopentanone or cyclohexanone to obtain a single chalcone-based reaction product, after separation and purification, react with the corresponding benzene Formaldehyde reacts to obtain the target substance.
Embodiment 3
[0327] Embodiment three, cyclic ketone derivatives 11 C radioactive isotope labeling method:
[0328]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com