Method for the serological diagnosis of rheumatoid arthritis
A specific, red blood cell technology, applied in chemical instruments and methods, disease diagnosis, immunoglobulin against animals/humans, etc., can solve problems such as limited sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: Preparation of citrullinated IgG
[0075] Rabbit IgG (25ug), citrullinated in vitro by rabbit skeletal muscle PAD (75mU; Sigma-Aldrich; EC 3.5.3.15) in deimination buffer (40mM Tris-HCl, pH 7.5, 5mM CaCl2 and 1mM DTT) Incubate at 37°C for 3h. The reaction was stopped by adding EGTA at pH 8.0 to a concentration of 50 mM. Citrullinated rabbit IgG was obtained after overnight dialysis against 10 mM Tris pH 7.6 with 2 mM EDTA.
[0076] Goat glycophorin A antibody (25ug), obtained from Abcam, UK, was prepared from rabbit skeletal muscle PAD (75mU; Sigma-Aldrich; EC 3.5.3.15) in deimination buffer (40mM Tris-HCl, pH 7.5, 5mM CaCl2 and 1 mM DTT) and incubated at 37°C for 3 h. The reaction was stopped by adding EGTA at pH 8.0 to a concentration of 50 mM. Citrullinated goat IgG was obtained after overnight dialysis against 10 mM Tris pH 7.6 with 2 mM EDTA.
[0077] Another glycophorin A antibody was obtained from Santa Cruz Biotechnology. R10sc-53905 is a mon...
Embodiment 2
[0079] Embodiment 2: Preparation of carbamylated IgG
[0080] Carbamylated IgG was obtained by incubating 1.18 mg of rabbit IgG dissolved in 1 mL of PBS with 1 mL of 0.2 M KCNO in 0.1 M Na2HPO4 buffer for three hours at 37 degrees Celsius. Carbamylated IgG was then dialyzed overnight at 4°C against 0.9% NaCl and stored at 4°C until use. This IgG was used as the antigen in an ELISA assay for the detection of anti-citrullinated antibodies in RA patient sera. Non-carbamylated IgG was used as a control therein.
Embodiment 3
[0081] Example 3: ELISA with citrullinated antibodies
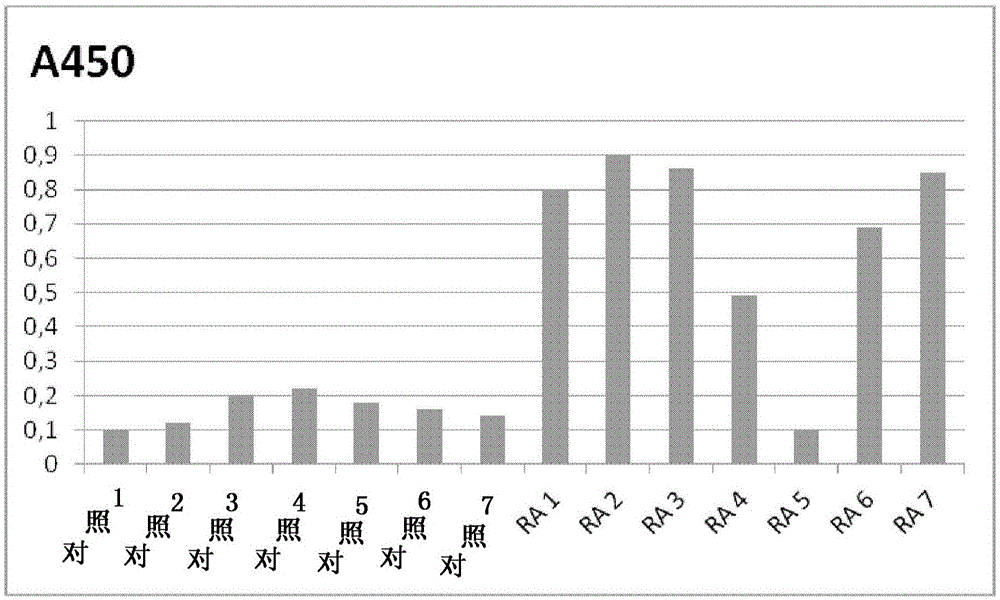
[0082] An ELISA assay to detect antibodies against citrullinated antibodies was set up as follows. Citrullinated IgG, prepared as described above, was immobilized on NUNCELISA plates by incubating 50 μl / well of 100 μg / ml citrullinated IgG overnight at 37°C. Remaining binding sites were performed by adding 50 microliters of 100 micrograms / ml bovine serum albumin (BSA) in phosphate-buffered saline (PBS) to each well of the microtiter plate and incubating for 1 hour at 37 degrees Celsius. block. The plates were then washed 3 times with PBS+0.05% Tween 20 (PBS / Tween) and stored dry at 4°C if not used immediately.
[0083] Control plates were prepared in exactly the same manner, except that they contained non-citrullinated antibodies from species-matched controls immobilized on the solid phase.
[0084] Serial dilutions (1 / 10 to 1 / 100.000) of human sera from RA patients known to be negative for rheumatoid factor were used t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com