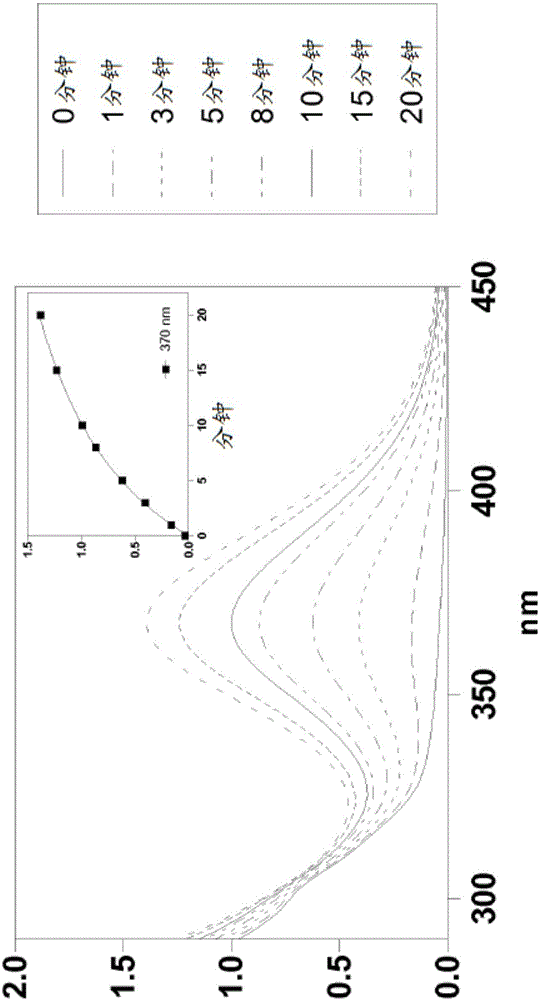
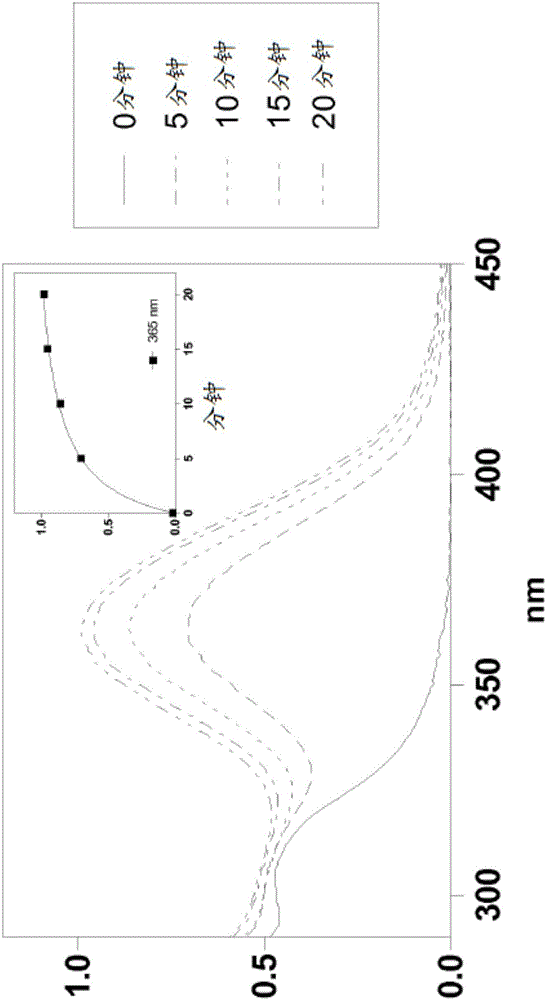
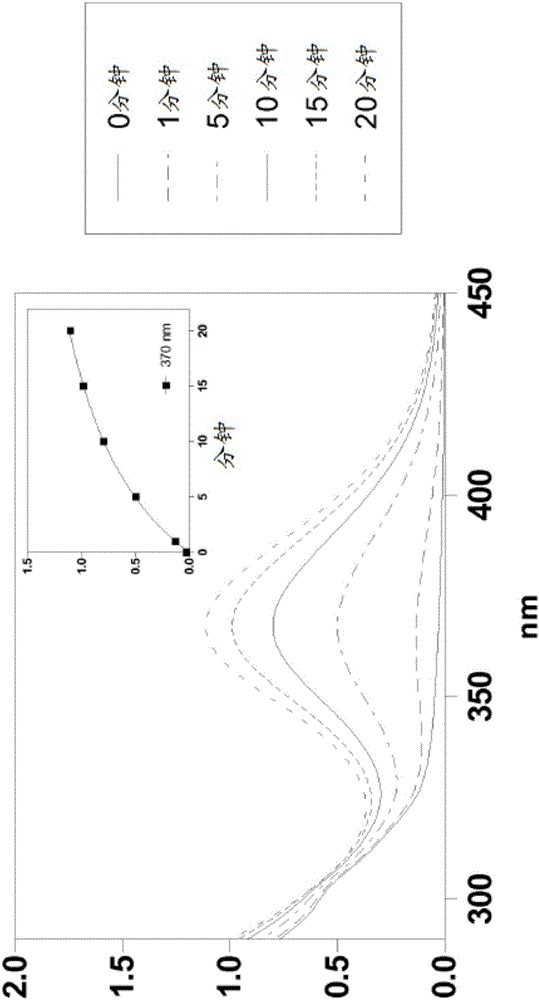
Silylated imine and carbamate polymeric benzoate compounds, uses, and compositions thereof
一种聚合物、组合物的技术,应用在周期表第4/14族元素的化合物、药物组合、化学仪器和方法等方向,能够解决减低保护效率、产率没有好、转化率低等问题,达到高UV保护作用、低制造成本的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Example 1. 4-((3-(triethoxysilyl)propylcarbamoyloxy)methyl)benzoic acid 3-(diethyl Synthesis of (amino)phenyl ester (compound 1)
[0228]
[0229] Step 1: Synthesis of 4-formylbenzoyl chloride
[0230] 0.92 g (6.13 mmol) of 4-formylbenzoic acid was suspended in 50 mL of anhydrous toluene. 16 mL of thionyl chloride (0.22 mol) was added and the resulting suspension was heated at 130° C. for 3 hours under a nitrogen atmosphere, then cooled to room temperature and the solvent was evaporated under reduced pressure. An additional 50 mL of toluene was added and evaporated under reduced pressure to eliminate possible remaining thionyl chloride. Repeat this process twice. The resulting solid was used without further purification.
[0231] Step 2: Synthesis of 3-(diethylamino)phenyl 4-formylbenzoate
[0232] 1.01 g (6.13 mmol) of 3-(diethylamino)phenol was suspended in 50 mL of dichloromethane and dissolved by adding 0.85 mL (6.13 mmol) of triethylamine. The resulting s...
Embodiment 2
[0240] Example 2. 4-((3-(triethoxysilyl)propylcarbamoyloxy)methyl)benzoic acid 3-(dimethyl Synthesis of (amino)phenyl ester (compound 2)
[0241]
[0242] Step 1: Synthesis of 4-formylbenzoyl chloride
[0243] 0.92 g (6.13 mmol) of 4-formylbenzoic acid was suspended in 50 mL of anhydrous toluene. 16 mL of thionyl chloride (0.22 mol) was added and the resulting suspension was heated at 130° C. for 3 hours under a nitrogen atmosphere, then cooled to room temperature and the solvent was evaporated under reduced pressure. An additional 50 mL of toluene was added and evaporated under reduced pressure to eliminate possible remaining thionyl chloride. Repeat this process twice. The resulting solid was used without further purification.
[0244] Step 2: Synthesis of 3-(dimethylamino)phenyl 4-formylbenzoate
[0245] 0.84 g (6.13 mmol) of 3-(dimethylamino)phenol was suspended in 50 mL of dichloromethane and dissolved by adding 0.85 mL (6.13 mmol) of triethylamine. The result...
Embodiment 3
[0253] Example 3. 3-Methoxy 4-((3-(triethoxysilyl)propylcarbamoyloxy)methyl)benzoate Synthesis of Phenyl Ester (Compound 3)
[0254]
[0255] Step 1: Synthesis of 4-formylbenzoyl chloride
[0256] 0.92 g (6.13 mmol) of 4-formylbenzoic acid was suspended in 50 mL of anhydrous toluene. 16 mL of thionyl chloride (0.22 mol) was added and the resulting suspension was heated at 130° C. for 3 hours under a nitrogen atmosphere, then cooled to room temperature and the solvent was evaporated under reduced pressure. An additional 50 mL of toluene was added and evaporated under reduced pressure to eliminate possible remaining thionyl chloride. Repeat this process twice. The resulting solid was used without further purification.
[0257] Step 2: Synthesis of 3-methoxyphenyl 4-formylbenzoate
[0258] 0.76 g (6.13 mmol) of 3-methoxyphenol was suspended in 50 mL of dichloromethane and dissolved by adding 0.85 mL (6.13 mmol) of triethylamine. The resulting solution was stirred for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com