Morphine derivative crystal form ii and its preparation method and use
A derivative, morphine technology, applied in the field of morphine derivative crystal form II and its preparation, can solve problems such as unstable placement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
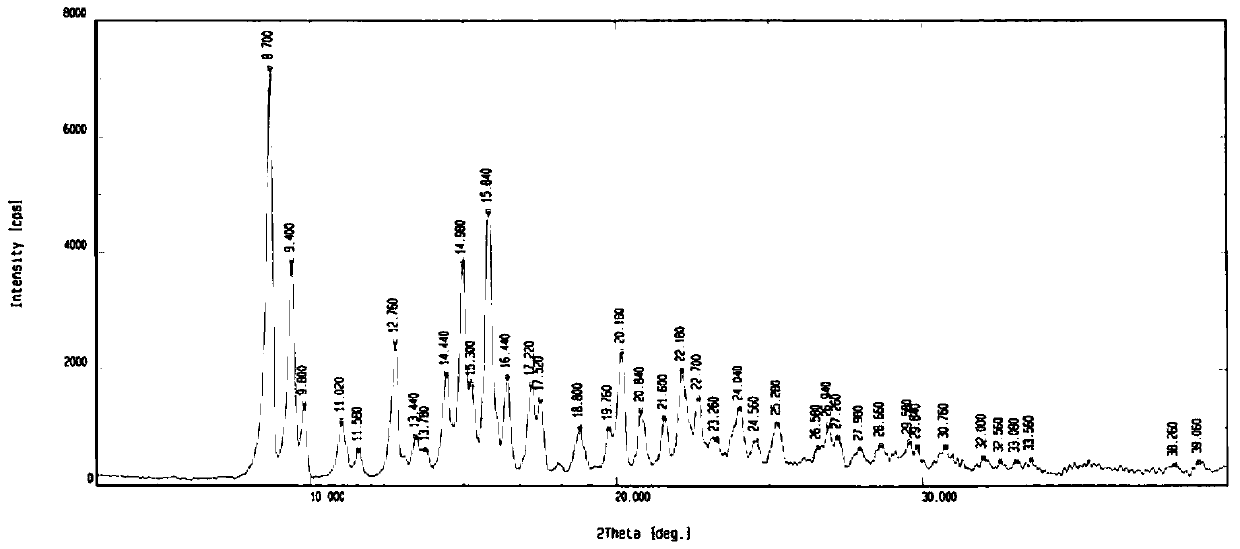
[0069] 1 g of the morphine derivative shown in Formula 1 (prepared according to the method described in CN201410116005.0) and 3 ml of acetone were prepared into a suspension, stirred at 5°C for 15 h, stirred and crystallized at 0°C for 10 h, filtered, and dried under reduced pressure at 60°C. The obtained morphine derivatives are 8.70, 9.40, 9.80, 11.02, 11.58, 12.76, 13.44, 13.78, 14.44, 14.98, 15.30, 15.84, 16.44, 17.22, 17.52, 18.80, 19.76, 20.18, 20.80, 21. There are absorption peaks at 22.18, 22.70, 23.26, 24.04, 24.56, 25.28, 26.58, 26.94, 27.26, 27.98, 28.66, 29.58, 29.84, 30.76, 32.00, 32.56, 33.08, 33.56, 38.26, and 39.06. Its X powder diffraction pattern is as figure 1 shown.
[0070] Morphine derivative crystal form II shown in crystal form 1 of the present invention, its powder X-ray pattern is represented by 2θ, d-value, I / I 0 Other parameters express the II crystal form, as shown in the table below:
[0071] 2θ°
d-value
I / I 0
8.70
...
Embodiment 2
[0077] Prepare a suspension of 1 g of the morphine derivative shown in Formula 1 and 5 ml of acetone, stir at 10°C for 5 h, stir and crystallize at 5°C for 1 h, filter, and dry under reduced pressure at 50°C. The obtained morphine derivative was identified by the characterization method in Example 1, and it was crystal form II.
Embodiment 3
[0079] Prepare a suspension of 1 g of the morphine derivative shown in Formula 1 and 10 ml of acetone, stir at 56°C for 11 h, stir and crystallize at 20°C for 15 h, filter, and dry under reduced pressure at 70°C. The obtained morphine derivative was identified by the characterization method in Example 1, and it was crystal form II.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition peak temperature | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com