Medicine composition for therapy of allergic rhinitis
A technology of allergic rhinitis and composition, applied in the field of medicine, can solve the problems of toxicity, side effects, high price, etc., achieve inhibition of IL-5 inhibition, good therapeutic effect, eliminate nasal cavity eosinophilia and The effect of mast cell activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
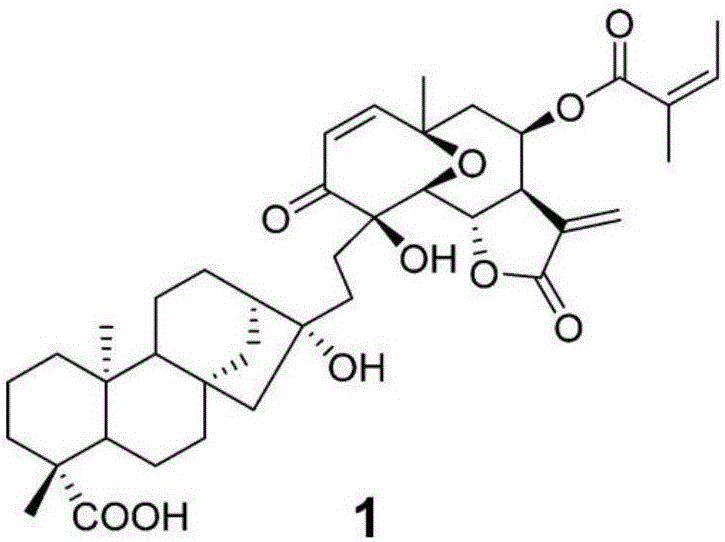
[0012] The above description is only an overview of the technical solution of the present invention. In order to understand the technical means of the present invention more clearly and implement it according to the content of the description and conventional technical means in the field, the animal test examples of the present invention will be further described in detail below .
[0013] The preparation method of the compound Helikaurolide A involved in the present invention can be found in literature (Helikaurolides A–D with a Diterpene-Sesquiterpene Skeleton from Supercritical Fluid Extracts of Helianthus annuus L.var.AriannaOrg.Lett., 2015, 17(19), 4730–4733).
[0014] Homoharringtonine, CAS: 6159-55-3. The market is available.
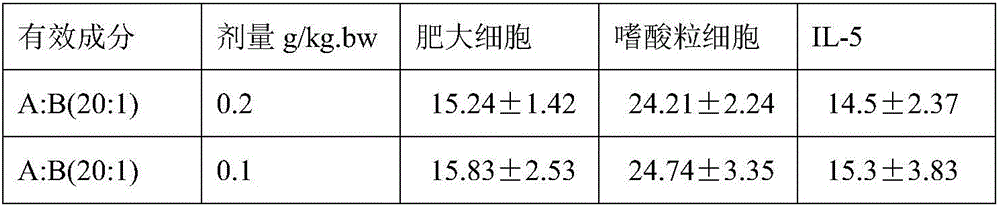
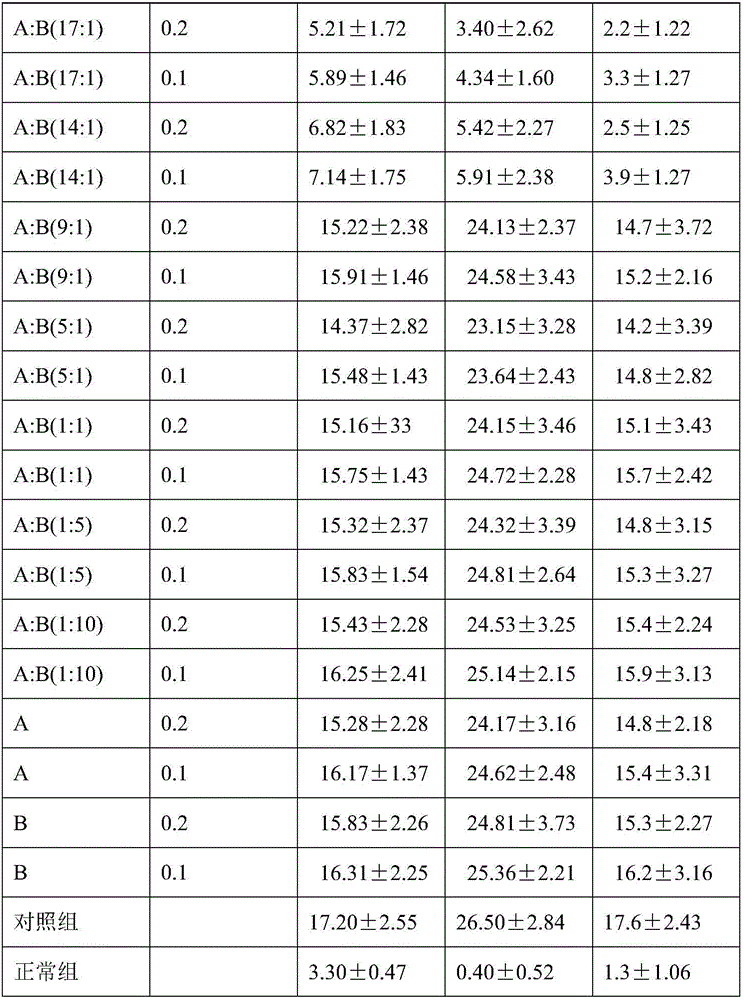
[0015] Modeling: clean-grade mice, male, weighing 22-28 g, the ambient temperature of the animal room is 27°C-30°C, and the humidity is 35%-45%. 220 mice were randomly divided into 22 groups, 10 in each group, divided into 20 groups of drug adm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com