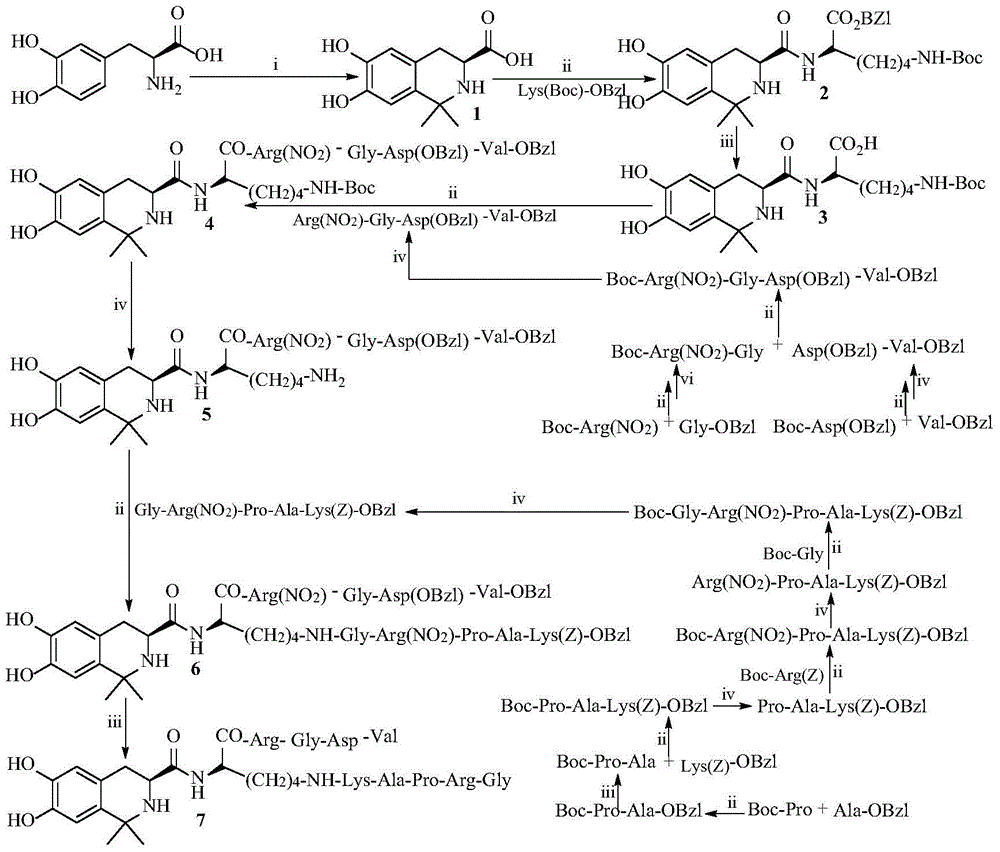
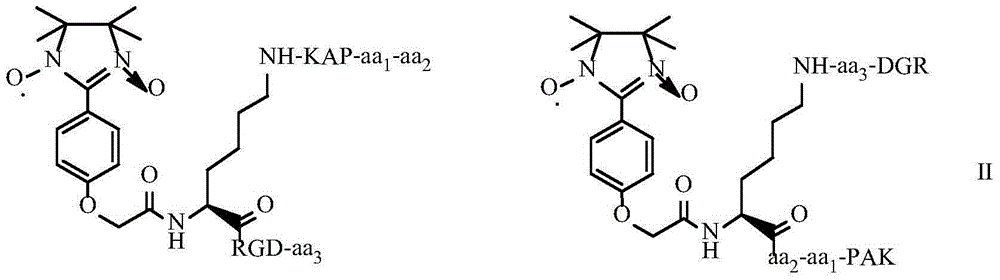
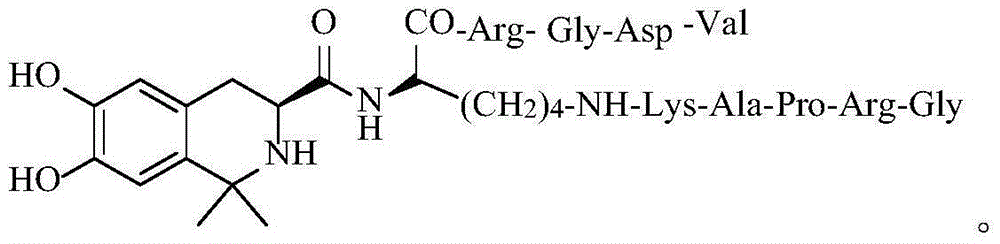Tetrahydroisoquinoline-3-formyl-K (GRPAK) RGDV and synthesis, activity and application thereof
A technology of tetrahydroisoquinoline and isoquinoline, applied in the preparation of antithrombotic drugs, thrombolytic drugs and drugs for the treatment of ischemic stroke, in the field of thrombolytic activity, can solve the problem of no effective drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 liquid phase connection peptide reaction general method
[0023] The N-terminal protected raw material was dissolved in anhydrous DMF, and N-hydroxybenzotriazole (HOBt) was added to the resulting solution. N,N-dicyclohexylcarbodiimide (DCC) dissolved in anhydrous DMF was slowly added under ice-cooling, and stirred at 0°C for 15 minutes to obtain a reaction solution (I). The carboxy-terminally protected raw material was also dissolved in anhydrous DMF, adjusted to pH 9 with N-methylmorpholine (NMM), then mixed with the reaction solution (I), maintained at pH 9 with N-methylmorpholine, and stirred at room temperature for 10 hour, TLC monitors that after the raw material point disappears, the reaction mixture is filtered, and the filtrate is concentrated under reduced pressure to obtain a viscous substance that is dissolved in ethyl acetate, and the solution obtained is successively washed with 5% NaHCO 3 Aqueous wash, 5% KHSO 4 Washing with aqueous solution...
Embodiment 2
[0024] Embodiment 2 removes Boc general method
[0025] The Boc-protected peptide was dissolved in a small amount of anhydrous ethyl acetate, and a solution of hydrogen chloride in ethyl acetate (4M) was added with stirring in an ice bath. After TLC showed that the raw material point disappeared, the reaction solution was repeatedly pumped dry with a water pump to remove hydrogen chloride gas, and the residue was repeatedly ground with petroleum ether or anhydrous ether to obtain the target compound.
Embodiment 3
[0026] Embodiment 3 debenzyl ester general method
[0027] Peptide benzyl ester with CH 3 OH was dissolved, and an aqueous solution of NaOH (2M) was slowly added dropwise with stirring in an ice bath. The temperature of the reaction solution was maintained at 0° C. in an ice bath, and TLC showed that the starting material disappeared. The reaction solution was adjusted to neutrality with 1M hydrochloric acid, methanol was removed under reduced pressure, and acidified to pH 2 with 1M hydrochloric acid, the solution was extracted with ethyl acetate, and the combined ethyl acetate phase was washed with saturated NaCl aqueous solution to neutrality, and washed with anhydrous Water Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain the target compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com