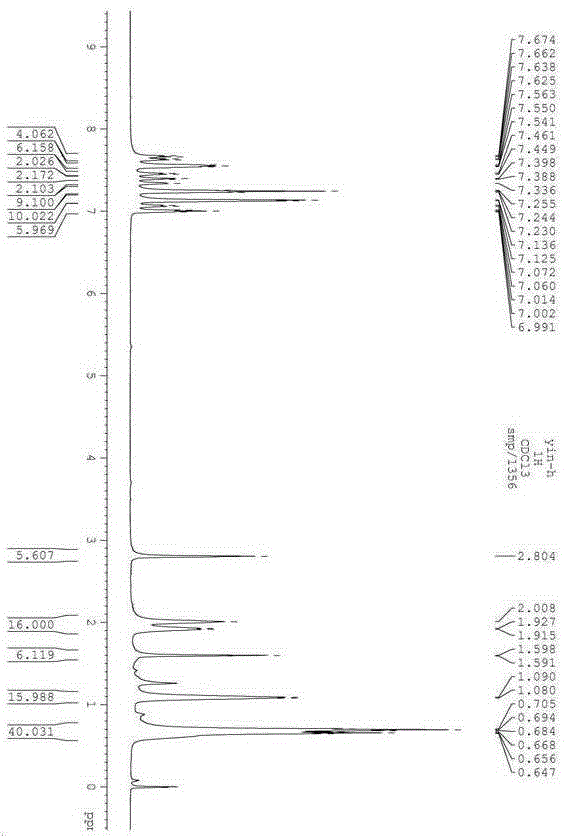
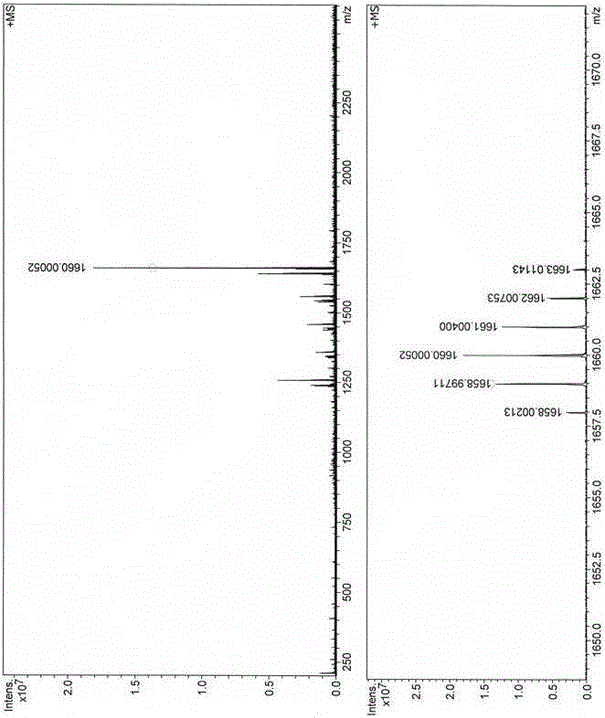
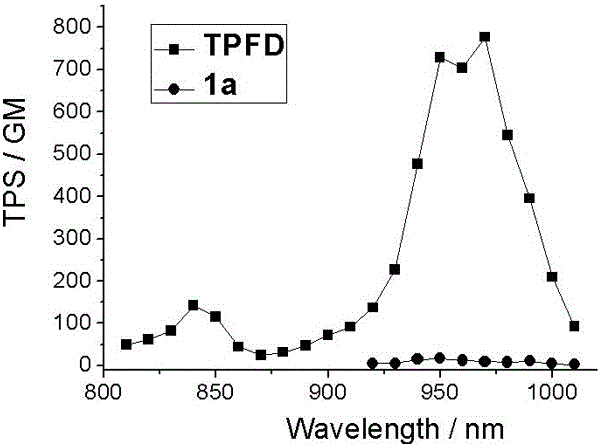
Two-photon fluorescent dye based on phenyl-substituted boron-dipyrromethene and diphenyl indenofluorene and synthetic method thereof
A technology for substituting fluoroboron dipyrrole and fluoroboron dipyrrole, which is applied in azo dyes, organic dyes, luminescent materials, etc., can solve the problem of insignificant improvement of the two-photon absorption cross-section, and achieves improved two-photon absorption cross-section and strong dual-photon absorption. Photonic fluorescence performance, the effect of high fluorescence quantum yield
Active Publication Date: 2017-05-10
ZHENGZHOU UNIV
View PDF1 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
For example, Wang et al. introduced alkynyl electron-donating groups at the 2 and 6 positions of fluoroboron dipyrrole, but the improvement of its two-photon absorption cross section was not obvious (29-60 GM)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0067] The brominated alkane in step B2 in embodiment 1 is n-bromobutane, it should be noted that when the number of carbon atoms of the brominated alkane is other C1~C3, C5~C18 brominated alkane except n-bromobutane , the present invention can also be implemented.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses two-photon fluorescent dye based on phenyl-substituted boron-dipyrromethene and diphenyl indenofluorene; the two-photon fluorescent dye has a structural general formula shown in the description, wherein R is C1-C18 alkyl; the synthetic steps include: using 2,4-dimethylpyrrole and benzaldehyde as raw materials to generate 5-phenyl-substituted and 2,6-iodide boron-dipyrromethene; reducing indenofluorene dione, alkylating, brominating, carrying out Pd(0) catalytic amination, carrying out Pd(0) and CuI catalyzed Sonogashira cross-coupling reaction, and removing trimethylsilyl to generate diphenylamine-indenofluorene-ethynyl compound; enabling the 2,6-iodide boron-dipyrromethene to react with the diphenylamine-indenofluorene-ethynyl compound to obtain the two-photon fluorescent dye; the two-photon fluorescent dye has high two-photon fluorescence, maximum two-photon absorption section in toluene reaches 776 GM, fluorescent quantum yield reaches 0.46, and a new idea to synthesize and apply two-photon fluorescent dyes based on boron-dipyrromethene is provided.
Description
technical field [0001] The invention belongs to the technical field of fluorescent dyes, and in particular relates to a two-photon fluorescent dye based on phenyl-substituted fluoroboron dipyrrole and dianilinoindenofluorene and a synthesis method thereof. Background technique [0002] Two-photon absorption is a third-order nonlinear optical effect. Organic materials with large two-photon absorption cross-sections have applications in three-dimensional optical storage, two-photon fluorescence imaging, two-photon optical limiting materials, and photodynamic therapy [4] and other fields have broad application prospects. In the research field of two-photon fluorescent dyes, the development of fluorescent dyes with larger two-photon absorption cross section is the key. [0003] Fluoroboron dipyrrole is a class of fluorescent chromophores with excellent performance. It has a large molar extinction coefficient, high fluorescence quantum yield, good photostability, good tolerance...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07F5/02C09K11/06C09B57/00
CPCC07F5/022C09B57/00C09K11/06
Inventor 郝新奇高翔菅宁歌贺新超曹筱妞朱新举赵雪梅宋毛平
Owner ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com