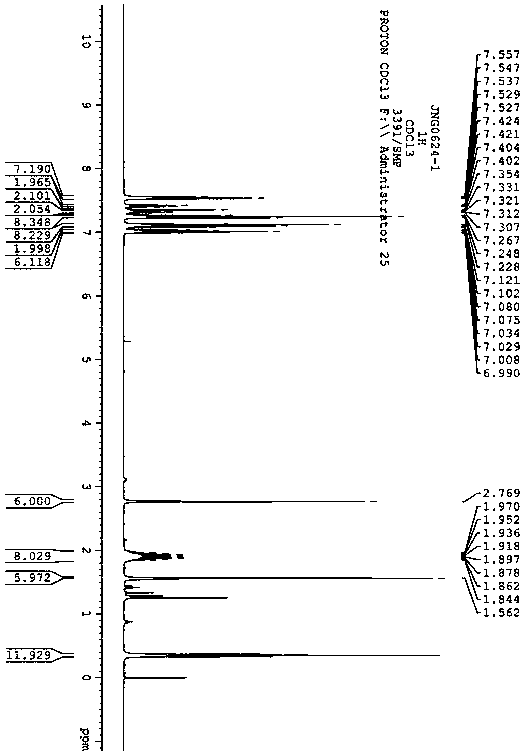
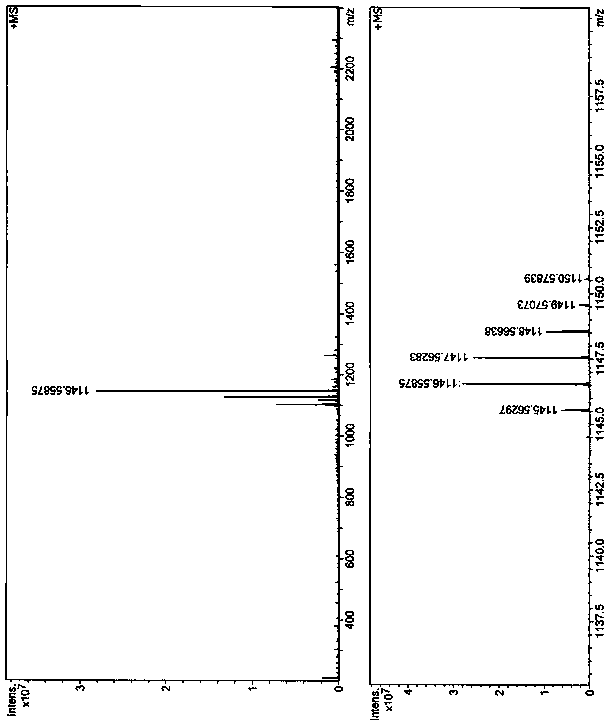
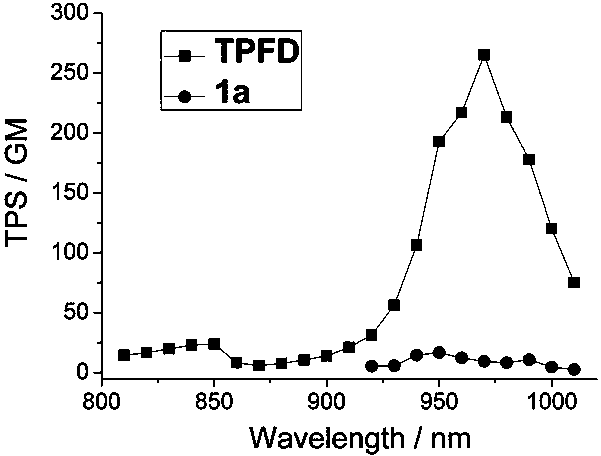
Two-photon fluorescent dye based on phenyl-substituted fluoroboron dipyrrole and dianilinofluorene and its synthesis method
A technology for substituting fluorine boron dipyrrole and fluorine boron dipyrrole, which is applied in the direction of azo dyes, organic dyes, luminescent materials, etc., can solve the problem that the improvement of the two-photon absorption cross section is not obvious, and achieve the improvement of the two-photon absorption cross section and high fluorescence Quantum yield, the effect of strong two-photon fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis route of the two-photon fluorescent dye based on phenyl-substituted fluoroborate dipyrrole and dianilinofluorene in this example is as follows:
[0039]
[0040] Among them, R is C 2 h 5 .
[0041] The synthesis method of the two-photon fluorescent dye based on phenyl-substituted fluoroborate dipyrrole and dianilinofluorene in this embodiment is as follows:
[0042] (1) Synthesis of 2,6-iodofluoroboron dipyrrole fluorophore 1a
[0043] A1: Under argon protection, add 500 mL of dichloromethane, 18.9 mmol of 2,4-dimethylpyrrole, 7.6 mmol of benzaldehyde and 0.1 mL of trifluoroacetic acid as catalysts into a 1000 mL three-neck flask, and stir magnetically for 6 h at room temperature ;
[0044] B1: Dissolve 7.6 mmol 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) in 150 mL of dichloromethane, add it to the reaction solution obtained in step A1, and continue stirring at room temperature for 15 min;
[0045] C1: Then add 10 mL diisopropylethylamine (DIEA) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com