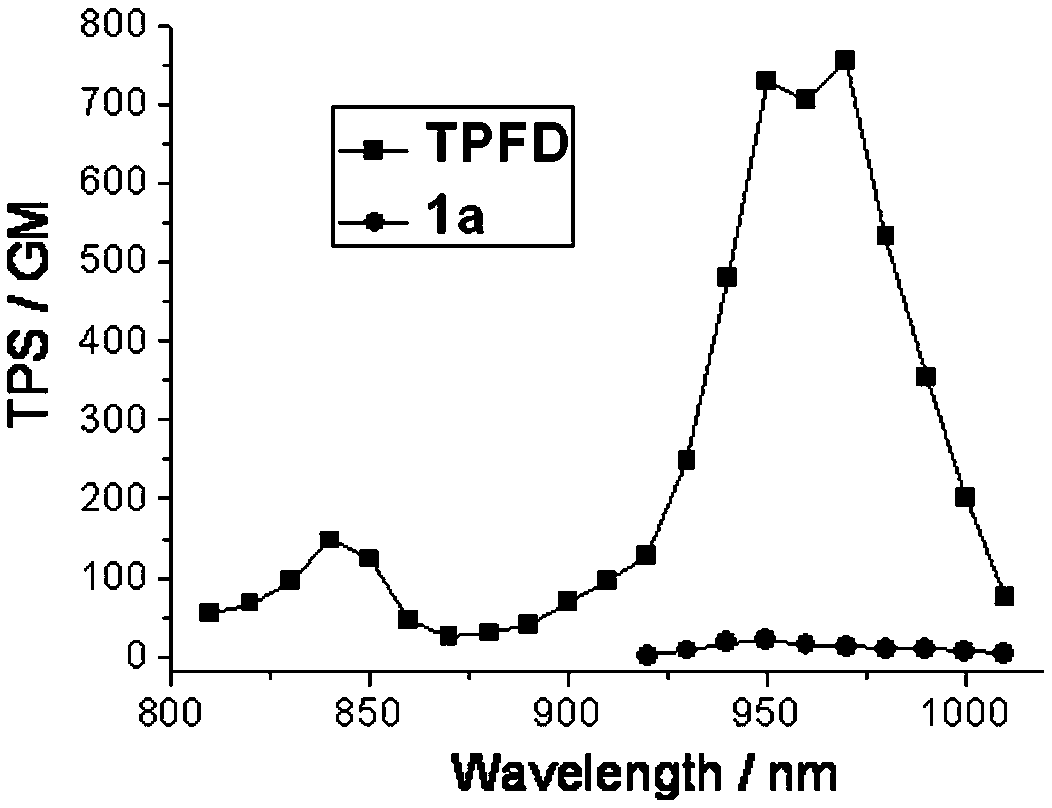
A two-photon fluorescent dye based on 4-methoxyphenyl substituted fluoroboron dipyrrole and dianilinoindenofluorene and its synthesis method
A fluoroboron-substituted dipyrrole, two-photon fluorescence technology, applied in the field of fluorescent dyes, can solve the problem of insignificant improvement of the two-photon absorption cross-section, achieve good single-photon and two-photon absorption and fluorescence performance, improve the two-photon absorption cross-section, The effect of high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
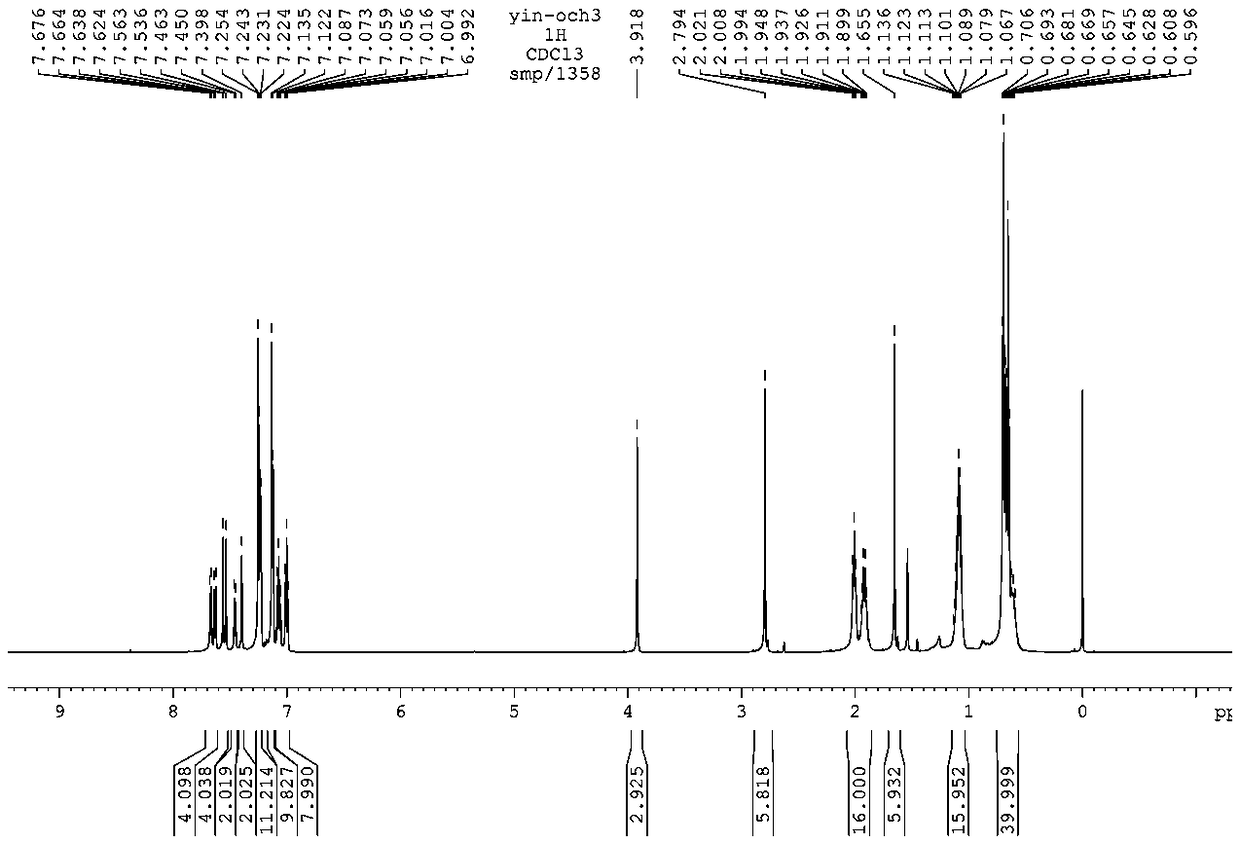
[0025] Example 1: Synthesis of 2,6-iodofluoroboron dipyrrole fluorophore 1a
[0026] Under argon protection, add 500 mL of dichloromethane, 18.9 mmol of 2,4-dimethylpyrrole, 7.6 mmol of 4-methoxybenzaldehyde and three drops of trifluoroacetic acid as catalysts into a 1000 mL three-neck flask, and stir magnetically at room temperature After 6 h, 7,9 mmol of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) was dissolved in 150 mL of dichloromethane, added to the above reaction solution, and continued to stir at room temperature for 15 min. Then, 10 mL of diisopropylethylamine (DIEA) and 10 mL of boron trifluoride ether solution were added to the above reaction liquid, and after stirring at room temperature for 30 min, 200 mL of water was added to quench the reaction, dichloromethane was extracted, and the liquids were separated. , dried over anhydrous sodium sulfate, the solvent dichloromethane was distilled off in vacuo, using dichloromethane:petroleum ether=1:2 as the eluent, and se...
Embodiment 2
[0028] Embodiment 2: the synthesis of diphenylamine-indenofluorene-acetylene compound 2f (R=C 4 h 9 )
[0029] (1) Synthesis of 2a
[0030] In a 250 mL three-neck round bottom flask, add 1.5 g (5.3 mmol) of indenofluorenedione, 120 mL of diethylene glycol, and then add 7.5 g of KOH and 8 mL of 98% hydrazine monohydrate. Then install a thermometer and a reflux condenser on the round-bottomed flask, slowly raise the temperature on a heating mantle with magnetic stirring to heat the temperature of the mixture to 160 °C, the temperature cannot continue to rise, and after heating and reflux for 24 h, change the device to a distillation device , distill off the water and excess hydrazine hydrate generated in the reaction, then continue to heat up, the temperature is raised to 196 ° C under reflux reaction for 24 h, after the reaction, pour the hot reaction solution into the ice-water mixture containing dilute hydrochloric acid , a solid was formed, let it stand for a while, the g...
Embodiment 3
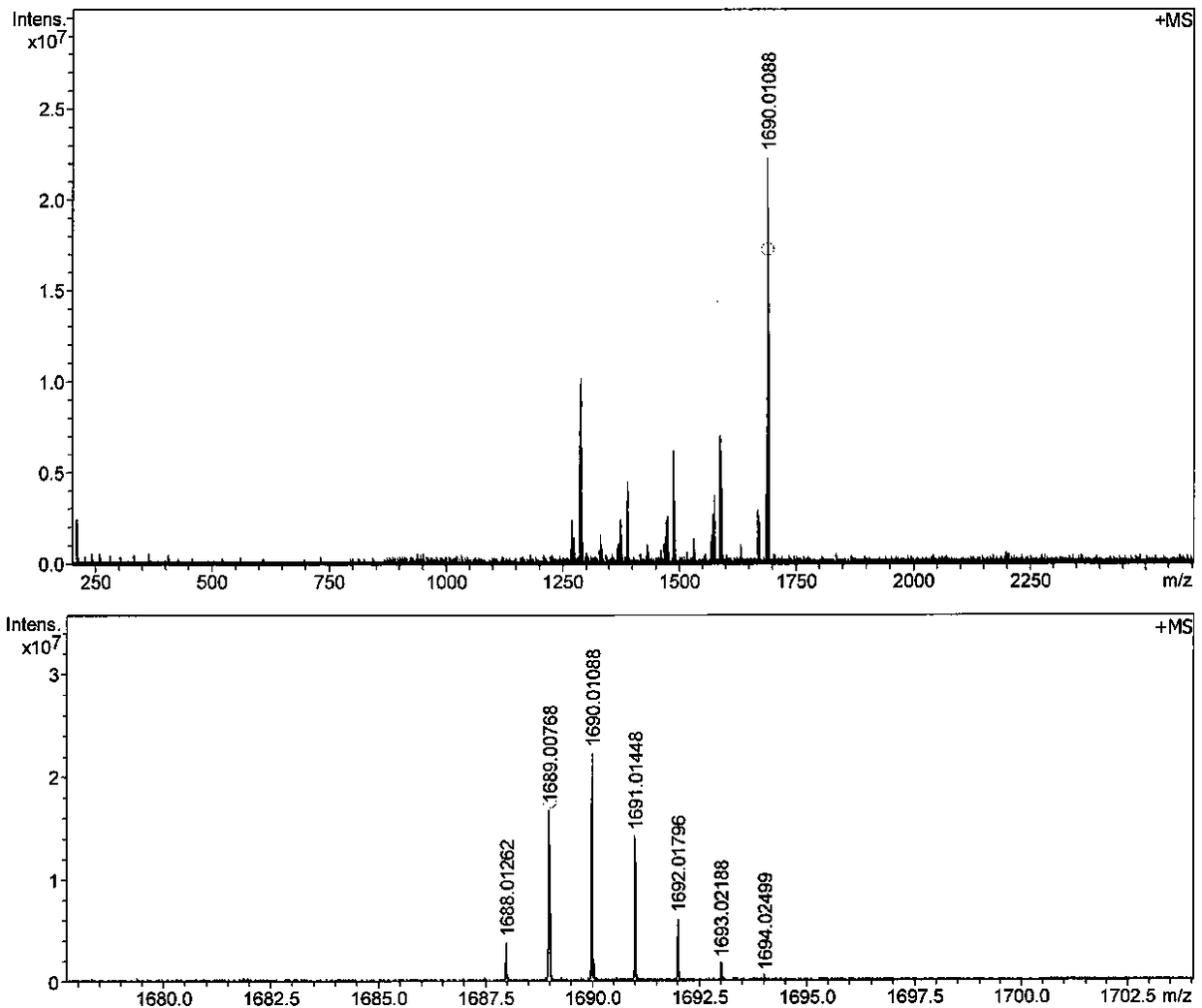
[0041] Embodiment 3: the synthesis of two-photon fluorescent dye TPFD (R=C 4 h 9 )
[0042] In a 10 mL Shrek reaction tube, add compound 2f (0.17 g, 0.26 mmol), Pd 2 (dba) 3 (4.58mg, 5%), tris(2-furyl)phosphine (2.32 mg, 10%) and cuprous iodide (1.9 mg, 10%), then add 0.8 mL of anhydrous DMF and 0.2 mL of freshly treated A mixed solvent of triethylamine, compound 1a (0.1 mmol) was added under the protection of nitrogen, and after reacting for 0.5 h at 60 °C, the TLC plate detected that the raw material fluoroborate dipyrrole had completely reacted, stopped the reaction, and poured the reaction solution into ether , transferred to a separatory funnel, washed several times with water, allowed to stand for stratification, dried the collected upper organic phase with anhydrous magnesium sulfate, filtered through a Buchner funnel to remove anhydrous magnesium sulfate, concentrated in vacuum on a rotary evaporator, The obtained blue solid was dissolved in dichloromethane and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com