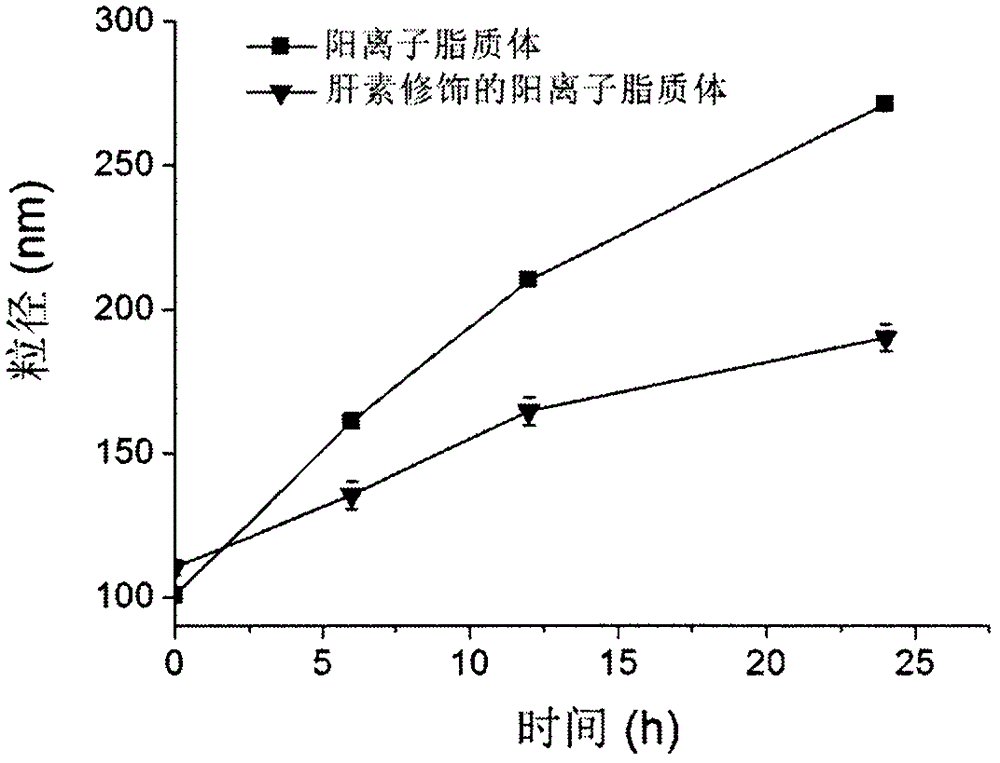
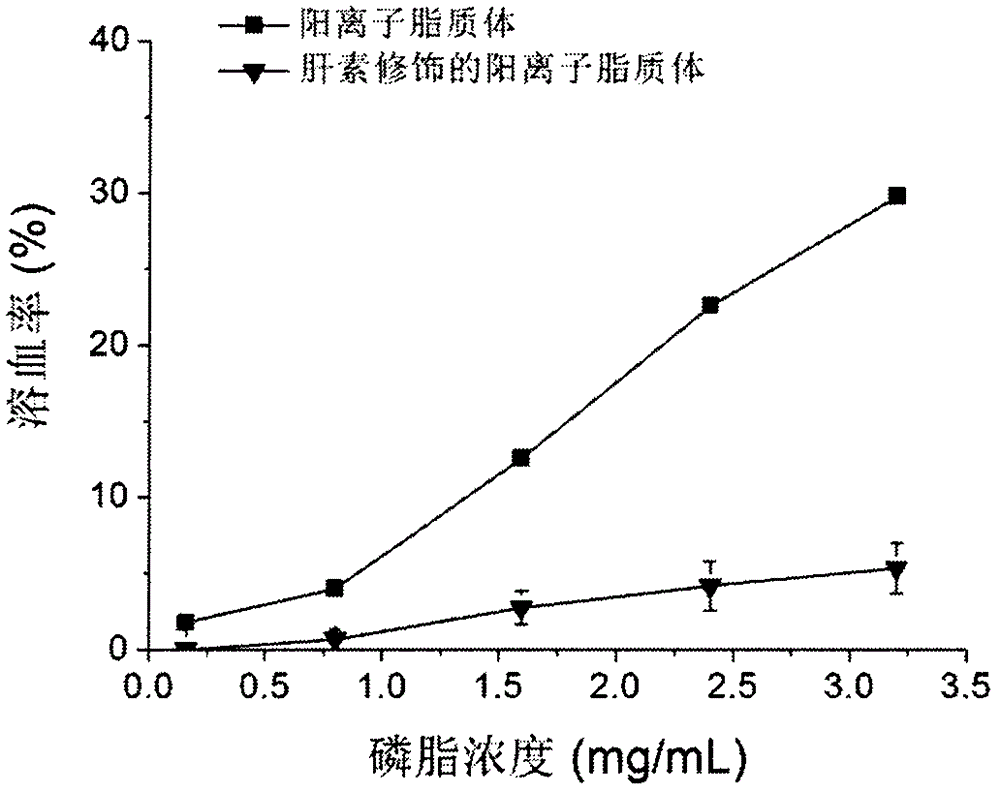
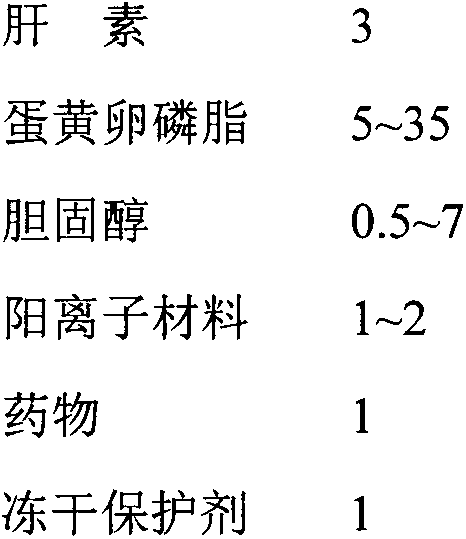
Heparin-modified cationic liposome and preparation method thereof
A technology of cationic liposomes and cationic lipids, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as limiting clinical applications and accelerating blood clearance, and achieve improved Effects of drug efficacy, safety improvement, and plasma stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The cationic liposome of embodiment 1 heparin modification
[0029] Weigh 10 mg of egg yolk lecithin, 1 mg of cholesterol, 1 mg of dioctadecyldimethylammonium bromide, and 1 mg of curcumin in the eggplant-shaped bottle, add an appropriate amount of dichloromethane, and ultrasonically dissolve them; rotate Evaporate under reduced pressure to remove the organic solvent, and form a dry and uniform lipid film on the bottle wall; add 1 mL of distilled aqueous solution to wash the film, and hydrate at 37 ° C for 1 hour to form a liposome primary emulsion; probe ultrasonic (200W, 100 times) to obtain Blank liposomes; add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and N-hydroxysulfosuccinimide to enoxaparin solution at a ratio of 1: 3. Activate enoxaparin; take cationic liposome solution and an equal volume of activated enoxaparin solution (4mg / mL) and stir overnight at room temperature; remove free heparin by ultrafiltration to obtain heparin-modified cationic l...
Embodiment 2
[0034] The cationic liposome of embodiment 2 heparin modification
[0035] Weigh 15 mg of egg yolk lecithin, 2 mg of cholesterol, 1 mg of stearylamine, and 1 mg of monodemethoxycurcumin in the prescribed quantities, respectively, and place them in an eggplant-shaped bottle, add an appropriate amount of chloroform, and ultrasonically dissolve them completely; Organic solvent, form a dry and uniform lipid film on the bottle wall; add 1mL of distilled aqueous solution to wash the film, hydrate at 37°C for 1 hour to form a liposome primary emulsion, and ultrasonic probe (200W, 100 times) to obtain a blank liposome . Take the above blank liposome and add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and N-hydroxysulfosuccinimide to the unfractionated heparin solution at a ratio of 1: 2. Activate unfractionated heparin; take cationic liposome solution and an equal volume of activated unfractionated heparin solution (4 mg / mL) and stir overnight at room temperature; remov...
Embodiment 3
[0037] Example 3 Heparin-modified cationic liposomes
[0038]Weigh 20 mg of egg yolk lecithin, 1.5 mg of cholesterol, 1 mg of trimethylhexadecyl ammonium bromide, 1 mg of bis-demethoxycurcumin, and place them in an eggplant-shaped bottle, add an appropriate amount of methanol, and ultrasonically dissolve them completely ; Remove the organic solvent by rotary decompression evaporation, and form a dry and uniform lipid film on the bottle wall; add 1 mL of distilled aqueous solution to wash the film, and hydrate at 37 ° C for 1 hour to form a liposome primary emulsion. Probe ultrasound (200W, 100 times), To obtain blank liposomes; take the above blank liposomes and add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxyl sulfosuccinimide to dalteparin solution, the ratio is 1:4, take the cationic liposome solution and an equal volume of dalteparin sodium solution (4mg / mL) and incubate for 30min at 25°C; remove free heparin by ultrafiltration to obtain the hep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap