Oncolytic adenovirus, carrier for preparing same and application thereof
An oncolytic adenovirus and adenovirus technology, applied in the field of biotechnology and gene therapy, can solve the problem that the efficiency of tumor-specific promoters cannot reach expectations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
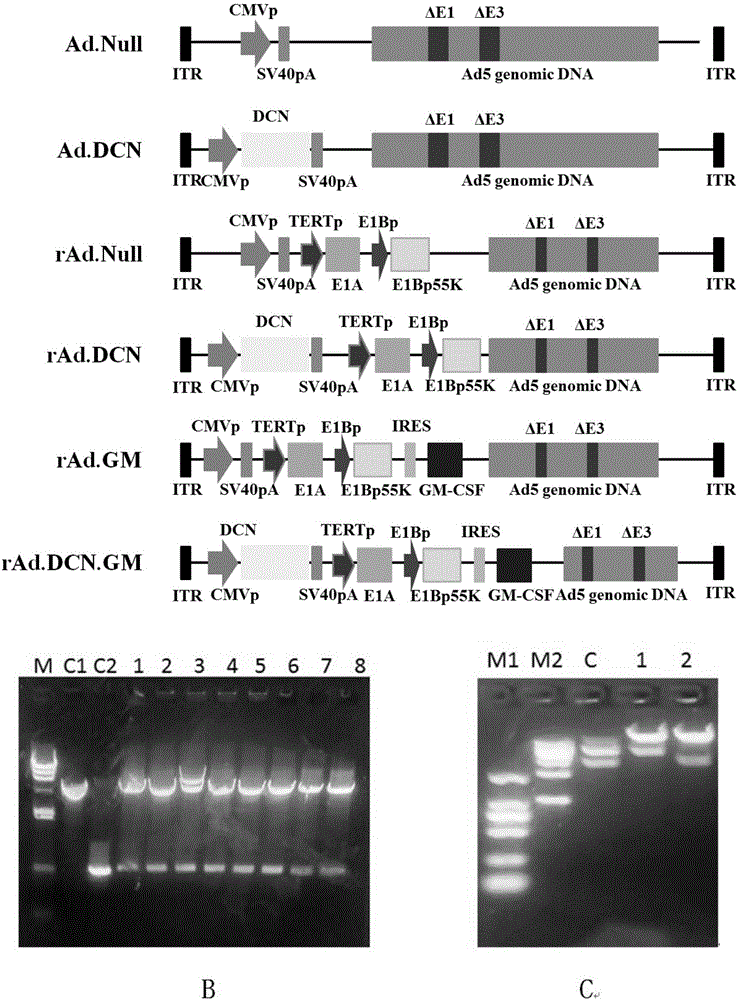
[0060] Example 1 Construction and preparation of recombinant adenovirus rAd.DCN
[0061] The method is to synthesize the human DCN gene, insert it into the pShuttle-CMV vector, and obtain pShuttle-CMV-DCN; digest the plasmid vector TE-TP-E1A-GM-55K preserved by our laboratory with MfeI, and obtain Gene, GM-CSF gene fragment - TERTp-E1A-E1Bp-GM-IRES-E1B55K, and clone it forward into the corresponding site of pShuttle-CMV-DCN to obtain the shuttle plasmid pSh.cmv.DCN.GM. RE; Refer to AdEasy system to obtain recombinant adenovirus plasmid pAd.DCN.GM.RE; and prepare oncolytic adenovirus rAd.DCN.GM from HEK293 cells. Specifically:
[0062] (1) Construction of pShuttle-CMV-DCN
[0063] Synthesize the full sequence of the human DCN gene through gene synthesis technology, and add BglII and XhoI enzyme cutting sites in the upstream and downstream, respectively, after digestion, connect to the corresponding site of pShuttle-CMV to obtain the shuttle carrying the target gene Plasmid p...
Embodiment 2
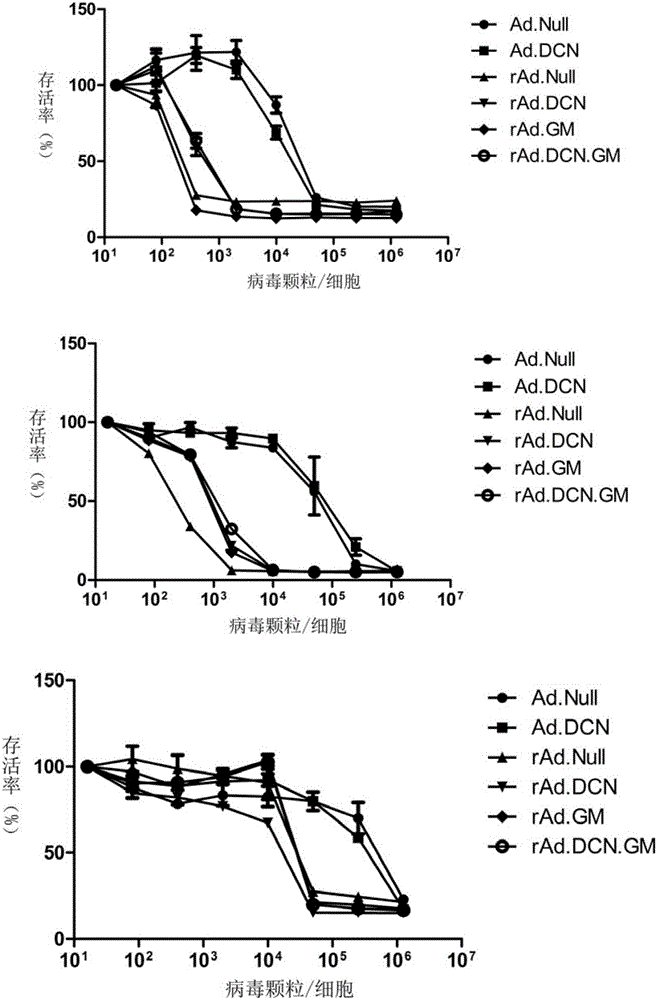
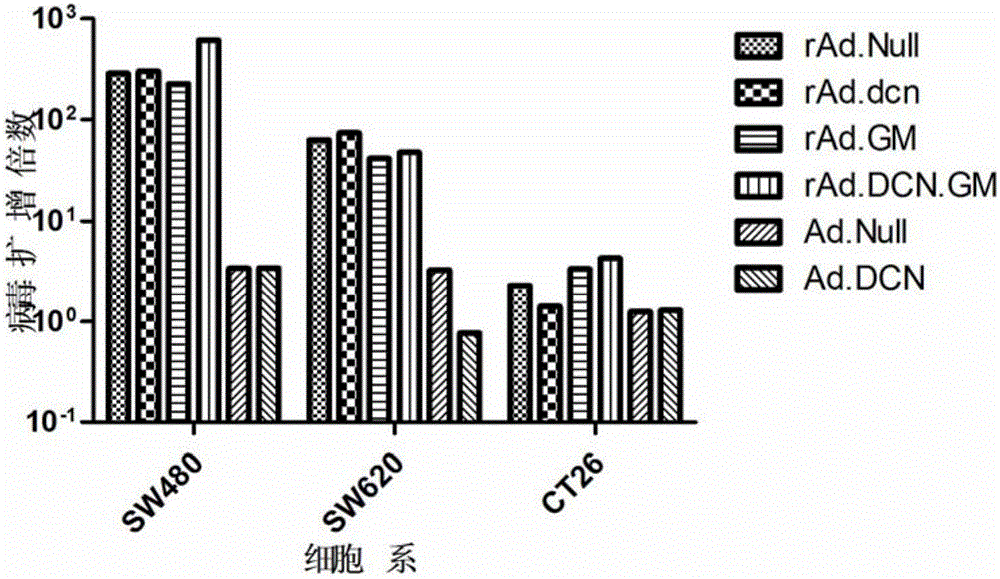
[0072] Example 2 In vitro function verification of oncolytic adenovirus rAd.DCN.GM
[0073] The method is to detect the killing effect of oncolytic virus in colon cancer cells by using sulforhodamine B (SRB) staining method; and to measure the oncolytic virus from 3h to 48h after infection by Adeno-X Rapid Titer Kit. The amplification factor of the virus in colon cancer cells was used to determine the replication ability of the virus; the expression of the target genes DCN and GM-CSF in colon cancer cells was detected by Western-blotting and ELISA. Specifically:
[0074] (1) Detection of killing ability of oncolytic adenovirus rAd.DCN.GM
[0075] Human kidney cancer cell lines SW620, SW480 and mouse colon cancer cell line CT26 were inoculated into 96-well plates at 1×103 cells / 100 μl medium / well, respectively. On the second day, the oncolytic adenovirus rAd.DCN, the control oncolytic viruses rAd.GM, rAd.DCN, rAd.Null, and the replication-defective control viruses Ad.Null and...
Embodiment 3
[0081] Example 3 Evaluation of curative effect of oncolytic adenovirus rAd.DCN.GM on colon cancer xenografts in mice
[0082] The method is to use mouse colon cancer cell CT26 to establish a mouse transplanted tumor model, administer oncolytic virus intratumorally for treatment, and monitor its growth to evaluate the effect of oncolytic virus on mouse kidney cancer transplanted tumors. Therapeutic effect. Specifically:
[0083] Mouse colon cancer cell CT26, Babl / c mice subcutaneously bearing tumor for 6-8 weeks with single cell suspension (2×106 cells / mouse), observe the growth of the tumor, and detect the growth of the tumor 8 days after the tumor bearing According to the volume, they were stratified and randomized into Buffer group, rAd.Null group, rAd.DCN group, rAd.GM group and rAd.DC.GM group. And the non-tumor-bearing mice were used as normal controls to evaluate the immune indexes.
[0084] On the 7th day after tumor bearing, oncolytic adenovirus was injected into th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com